Assignment Help With Gas Reservoirs
Gas Reservoirs
 In general, if the reservoir temperature is above the critical temperature of the hydrocarbon system, the reservoir is classified as a natural gas reservoir. On the basis of their phase diagrams and the prevailing reservoir conditions, natural gases can be classified into four categories:
In general, if the reservoir temperature is above the critical temperature of the hydrocarbon system, the reservoir is classified as a natural gas reservoir. On the basis of their phase diagrams and the prevailing reservoir conditions, natural gases can be classified into four categories:
- Retrograde gas-condensate
- Near-critical gas-condensate
- Wet gas
- Dry gas
Retrograde gas-condensate reservoir
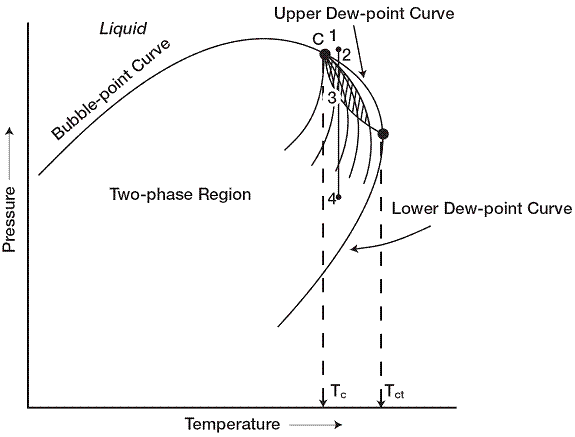
If the reservoir temperature T lies between the critical temperature Tc and cricondentherm Tct of the reservoir fluid, the reservoir is classified as a retrograde gas-condensate reservoir. This category of gas reservoir is a unique type of hydrocarbon accumulation in that the special thermodynamic behavior of the reservoir fluid is the controlling factor in the development and the depletion process of the reservoir. When the pressure is decreased on these mixtures, instead of expanding (if a gas) or vaporizing (if a liquid) as might be expected, they vaporize instead of condensing. Because the reservoir pressure is above the upper dew-point pressure, the hydrocarbon
![]()
system exists as a single phase (i.e., vapor phase) in the reservoir. As the reservoir pressure declines isothermally during production from the initial pressure to the upper dew-point pressure, the attraction between the molecules of the light and heavy components cause them to move further apart further apart. As this occurs, attraction between the heavy component molecules becomes more effective; thus, liquid begins to condense. Further reduction in pressure permits the heavy molecules to commence the normal vaporization process. This is the process whereby fewer gas molecules strike the liquid surface and causes more molecules to leave than enter the liquid phase. The vaporization process continues until the reservoir pressure reaches the lower dew-point pressure. This means that all the liquid that formed must vaporize because the system is essentially all vapors at the lower dew point
It should be recognized, however, that around the well bore where the pressure drop is high, and enough liquid dropouts might accumulate to give two-phase flow of gas and retrograde liquid.
The associated physical characteristics of this category are:
- Gas-oil ratios between 8,000 to 70,000 scf/STB. Generally, the gas-oil ratio for a condensate system increases with time due to the liquid dropout and the loss of heavy components in the liquid.
- Condensate gravity above 50° API
- Stock-tank liquid is usually water-white or slightly colored.
There is a fairly sharp dividing line between oils and condensates from a compositional standpoint. Reservoir fluids that contain heptanes and are heavier in concentrations of more than 12.5 mol% are almost always in the liquid phase in the reservoir. Oils have been observed with heptanes and heavier concentrations as low as 10% and condensates as high as 15.5%. These cases are rare, however, and usually have very high tank liquid gravities.
Near-critical gas-condensate reservoir
If the reservoir temperature is near the critical temperature the hydrocarbon mixture is classified as a near-critical gas-condensate. The volumetric behavior of this category of natural gas is described through the isothermal pressure declines and also by the corresponding liquid dropout curve. Because all the quality lines converge at the critical point, a rapid liquid buildup will immediately occur below the dew point as the pressure is reduced. This behavior can be justified by the fact that several quality lines are crossed very rapidly by the isothermal reduction in pressure. At the point where the liquid ceases to build up and begins to shrink again, the reservoir goes from the retrograde region to a normal vaporization region
Wet-gas reservoir
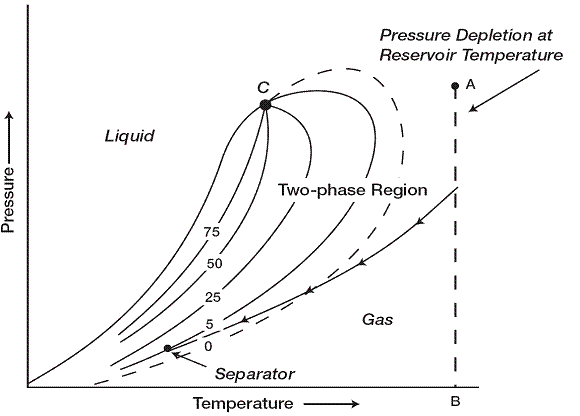
A typical phase diagram of a wet gas is shown in where reservoir temperature is above the cricondentherm of the hydrocarbon mixture. Because the reservoir temperature exceeds the cricondentherm of the hydrocarbon system, the reservoir fluid will always remain in the vapor phase region as the reservoir is depleted isothermally. As the produced gas flows to the surface, however, the pressure and temperature of the gas will decline. If the gas enters the two-phase region, a liquid phase will condense out of the gas and be produced from the surface separators. This is caused by a sufficient decrease in the kinetic energy of heavy molecules with temperature drop and their subsequent change to liquid through the attractive forces between molecules.
![]()
Wet-gas reservoirs are characterized by the following properties
- Gas oil ratios between 60,000 to 100,000 scf/STB
- Stock-tank oil gravity above 60° API
- Liquid is water-white in color
- Separator conditions, i.e., separator pressure and temperature, lie within the two-phase region.
Dry-gas reservoir
The hydrocarbon mixture exists as a gas both in the reservoir and in the surface facilities. The only liquid associated with the gas from a dry-gas reservoir is water. Usually a system having a gas-oil ratio greater than 100,000 scf/STB is considered to be a dry gas.
Kinetic energy of the mixture is so high and attraction between molecules so small that none of them coalesce to a liquid at stock-tank conditions of temperature and pressure. It should be pointed out that the classification of hydrocarbon fluids might be also characterized by the initial composition of the system.
Email Based Assignment Help in Gas Reservoirs
To submit Gas Reservoirs assignment click here
Following are some of the topics in General Composition Of Petroleum in which we provide help:
- General Composition Of Petroleum
- Physical Properties Of Hydrocarbons
- Origin of Petroleum
- Fundamental properties Of Fluid Permeated Rocks
- Porosity
- Permeability
- The Klinkenberg Effect
- Saturation
- Wettability
- Capillary Pressure
- Relative Permeability
- Drainage Process
- Three phase Relative Permeability
- Rock Compressibility
- Fundamentals Of Reservoir Fluid Behavior
- Classification Of Reservoir And Reservoir Fluids
- Gas Reservoirs
- Fundamentals Of Reservoir Fluid Flow
- Types Of Fluids
- Properties Of natural Gases
- Behavior Of Ideal Gases
- Behavior of Real Gases
- Compressibility Of Natural Gases
- Properties Of Crude Oil Systems
- Gas Solubility
- Determination And Application of Reservoir Fluid Properties
- Composition Of The Reservoir Fluid
- Differential Liberation Test
- Separator Tests
- Fluid Analysis Data On Gas
- Constant-Volume Depletion
- Oil Recovery mechanisms And The material Balance Equation
- Primary Recovery Mechanisms
- The Depletion Drive Mechanism
- Gas Cap Drive
- The Water Drive Mechanism
- Water Production
- The Gravity-Drainage-Drive Mechanism
- The Combination-Drive Mechanism
- The Material Balance Equation
- Change in Pore Volume Due to Initial Water and Rock Expansion
- Gas Reservoirs Help
- The Volumetric Method
- The material Balance Method


