Chemistry Assignment Help with Voids

10.9 Voids
In crystal lattice, following are the main voids
Linear void
This type of void is formed when two large atoms (anions) are arranged linearly. A small atom (cation) can occupy this void.
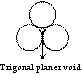
Trigonal planer void
Void space among three atoms of same size in a plane is known as trigonal planer void
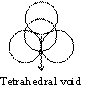
Tetrahedral planer void
If one atom is placed over three other atoms which are in contact, then space available among four atoms is called tetrahedral void.
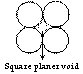
Square planer void
Void space available between four atoms in contact is square planer void
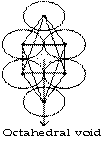
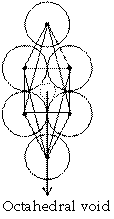
Octahedral void
It we put four atoms in a plane which are in contact and put one atom above and one below then, void available among six atoms is called octahedral void
Cubical void
If we put four atoms in one plane which are in contact and put another four atom over it, then space available is cubical void.

Tetrahedral void in fcc or ccp
In the given fc.c. unit cell, it we consider any corner then there will be three faces associated with it. Tetrahedral void is formed by four atoms, three from faces and one from corner. Because there are eight centres in unit cell hence there will be eight tetrahedral voids. If we divide the unit cell into eight equal small cubes, then centre of small cube will correspond to the centre of tetrahedral void from given figure let us consider two specified cubes A and B. By considering these cubes we can prove the location of tetrahedral void
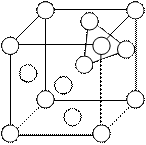
Octahedral voids in fcc
For octahedral void consider any four atoms from four faces, these will construct a square planer void, now if we put other two face atoms above and below to the four atoms, then these six will form octahedral void. Apart from the centre octahedral voids are also present at edge centre, each edge centred octahedral void contributes ¼ of its volume to the unit cell. Hence total contribution to the unit cell will be
![]()
Tetrahedral and octahedral voids in hcp
In hcp total number of tetrahedral voids = 12
In hcp total number of octahedral voids = 6

Email Based Assignment Help in Voids
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Voids in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Voids. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Voids assignment click here.


