Chemistry Assignment Help with Radius Ratio
- Home
- Chemistry
- Solid State
- Radius Ratio

10.10 Radius Ratio
In ionic solid the central cation can touch only that number of anions which should lead to maximum electrostatic attraction and minimum electrostatic repulsion. These interactions depend on size of anion and cation and are greatly influenced by the ratio of radius of the cation and the anion.
Radius ratio <![]()
Larger the ratio, greater will be the number of anions in surrounding and greater will be its co-ordination number
Linear void (CN = 2)
Radius ratio for C.N = 2 is less then 0.155 or ![]()
Trigonal planer void (CN = 3)
Let us consider three anions in contact and relatively small cation is present in void. Centre of cation and anions are in the same plane. An equilateral triangle can be constituted by joining centres of anions. Orthocenter of triangle and centre of cation are common. By considering the figure.![]()
![]()
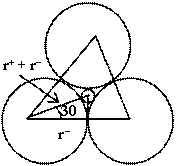
or ![]()
or ![]()
hence for trigonal planer void
0.155 ![]()
![]() < 0.225
< 0.225
Tetrahedral void (CN = 4)
Radius ratio for tetrahedral void
0.225![]()
Square planer (CN = 4)/Octahedral void (CN = 6)
Radius ratio for square planer and octahedral voids
0.414![]()
Cubical void (CN = 8)
Radius ratio for cubical void ![]()

Email Based Assignment Help in Radius Ratio
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Radius Ratio in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Radius Ratio. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Radius Ratio assignment click here.


