Chemistry Assignment Help with Pressure Volume Work
7.6 Pressure-Volume Work
7.6.1 Work Done in Reversible Isothermal Expansion


dw = – (Pext – dp). dV
= – Pext. dV = – PdV
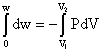
 w =
w = 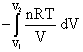
w = – nRT ln ![]()
w = – 2.303 nRT log ![]()
Since temp is constant therefore P1V1=P2V2 ![]()
wrev = – 2.303 nRT log ![]()
7.6.2 Irreversible Isothermal Process
w = – Pext(V2 – V1)
Pext is less than the pressure of the gas, the work done during intermediate expansion is numerically less than during reversible isothermal expansion.
7.6.3 Reversible Adiabatic Process
Work done = ![]()

Email Based Assignment Help in Pressure Volume Work
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Pressure Volume Work in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Pressure Volume Work. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Pressure Volume Work assignment click here.


