Chemistry Assignment Help with Mosley Experiment
3.4 Mosley Experiment

Moseley a brilliant student of Rutherford, in 1913 made the first detailed study of the characteristic x-ray spectrum emitted by different elements and showed that the square root of the frequency (![]() ) of a spectral line is directly related with the nuclear charge (Z), if the excitation potential is fixed.
) of a spectral line is directly related with the nuclear charge (Z), if the excitation potential is fixed.
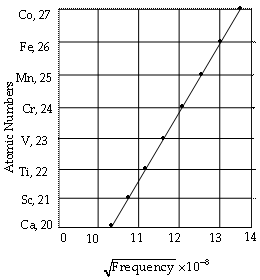
When a graph was plotted between ![]() × 10–8 and the atomic number (Z), a straight line was obtained.
× 10–8 and the atomic number (Z), a straight line was obtained.
The results obtained led to the suggestion that (![]() ) must be directly proportional to the atomic number of an element.
) must be directly proportional to the atomic number of an element.
To give accurate results, Mosley modified this equation as
where b is a constant and is known as screening constant. For Ka and Kb lines, b = 1.
Hence,
where a is proportionality constant.

Email Based Assignment Help in Mosley Experiment
Assignmenthelp.net provides email based Assignment Help, homework help in chemistry all topics. Our online tutors are available to help you in chemistry Mosley Experiment topics.
To submit chemistry Mosley Experiment assignment Click here.


