Chemistry Assignment Help with Radioactivity
- Home
- Chemistry
- Chemical Kinetics
- Radioactivity
4.9 Radioactivity
All radioactive decay follow 1st order kinetics and this is where the similarity ends. The rate of radioactive reactions is measured by calculating the rate of change of nuclei of the radioactive substance. For a radioactive decay A ® B, the rate of reaction is calculated as![]()
Integrating the differential rate law we get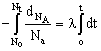

log ![]() Where No = number of nuclei of A at t = 0
Where No = number of nuclei of A at t = 0
Nt = number of nuclei of A at t = t
l = decay constant
The expression can be rearranged to give
Nt = N0e–lt …(1)
This suggests that the number of nuclei of radioactive substance A at any instant of time can be calculated, by knowing the number of nuclei at t = 0, its decay constant and the time.
Half-Life
Just like a 1st order reaction the half life of radioactive decay is given by![]()
Average–Life Time
Average life time is defined as the life time of a single isolated nucleus. The life time of a single isolated nucleus is ![]() .
.![]() .
.

Email Based Assignment Help in Radioactivity
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Radioactivity in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Radioactivity. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Radioactivity assignment click here.


