Chemistry Assignment Help with Factors Affecting Equilibrium Constant
5.5 Factors Affecting Equilibrium Constant
Representation of Equilibrium Reaction
Though equilibrium can be attained from either side, the way equilibrium is represented is important for equilibrium constant calculation.Example : - For
equilibrium constant
while for

Thus
Hence it is important how equilibrium is represented in equation.
Stoichiometry of Equilibrium Reaction
For reaction
and for
Though two equilibrium reaction are same representing change in same direction. Two equilibrium constants are not same. In fact
Thus stoichiometry equation is important
Activity Representation
Equilibrium constant value changes by the way activity of reactant/ products is represented. Equilibrium constant of concentration is not equal to equilibrium constant of pressure if numbers of moles are changing in reversible reaction.
Reaction Quotient
In a reversible reaction; concentration of Reactants and products keep on changing, till equilibrium is established. At any point of time the ratio of activity of Reactant(s)/ Product(s)
For Reaction
The value of reaction quotient helps to predict state of reversible reaction.
If Q > Kc then the rate of backward reaction is faster then forward reaction
If Q = Kc then the reaction is in equilibrium i.e., rate of forward reaction is equal to rate of backward reaction.
If Q < Kc then, the rate of forward reaction is greater then backward reaction.
Relation between Kc & Kp
For the process
If we consider partial presence of all species then by gas equation
PV=nRT
or P = CRT
Thus PA = CART
PB = CBRT
PN= CNRT
PM = CMRT
Equilibrium constant
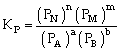
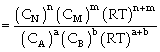
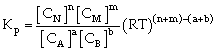
or
Where

Email Based Assignment Help in Factors Affecting Equilibrium Constant
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Factors Affecting Equilibrium Constant in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Factors Affecting Equilibrium Constant. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Factors Affecting Equilibrium Constant assignment click here


