Rutherford's Scattering Experiment Assignment Help
- Home
- Chemistry
- Atomic Structure
- Rutherford's Scattering Experiment
Rutherford's Scattering Experiment
In 1911 Ernst Rutherford (1871-1937) carried out a series at experiments using a-particles. A beam of a-particles was directed against a thin foil of about 0.0004 cm thickness of gold, platinum, silver or copper. The following observations were made:
- Most of the a-particles (more than 99%) went straight without suffering any deflection.
- A few of them got deflected through small angles.
- A very few (approximately 0.005%) did not pass through the foil at all but suffered large deflections (more than 90°) or even come back in more or less the direction from which they have come.
Following conclusion were drawn from the above observations.
 (a).Since most of the a-particles went straight through the metal foil undeflected, it means that there must be very large empty space with in the atom.
(a).Since most of the a-particles went straight through the metal foil undeflected, it means that there must be very large empty space with in the atom.(b).A few of the a-particles were deflected from their original paths through moderate angles; it was conclude that whole of the positive charge is concentrated and the space occupied by this positive charge is very small in the atom. When a-particles come closer to this point, they suffer a force of repulsion and deviate from their paths.
The positively charged heavy mass which occupies only a small volume in an atom is called nucleus. It is supposed to be present at the centre of the atom.
(c).A very few of the a-particles suffered strong deflections or even returned on their path indicating that the nucleus is rigid and a-particles recoil due to direct collision with the heavy positively charged mass.Rutherford concluded that the number of particles N scattered at an angle q is such that
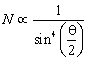
Later Rutherford concluded that mass number is twice the nuclear charge this forced him to modify his own model as ‘Atomic nucleus consists of protons and enough electrons to reduce the positive charge to about half the mass number and the remaining positive charge on the nucleus was balanced by the planetary electrons’.
Rutherfor'ds Scattering Experiment Assignment Help By Online Tutoring and Guided Sessions at AssignmentHelp.Net
Customer Support Services:
Assignmenthelp.net - Our expert Physics tutors are ready to help you round-the-clock (24/7 Live Supports by Phone Chat or Email (support@assignmenthelp.net). We provide plagiarism free Physics/Chemistry Courses to the students and also offer free download facilities of physics and chemistry tutorials as well.
To submit Physics/Chemistry Courses Click here



