EP204 Introduction to Material and Energy Balances
{`FACULTY OF ENGINEERING
UCSI University
CourseCode & Name: EP204 Introduction to Material and Energy Balances
`}
Q1 Methyl acetate, C3H6O2 (SG = 0.933) is mixed with acetone, C3H6O (SG = 0.791) in one of the processes in a chemical plant. The two liquids are blended until a mixture with a specific gravity of 0.85 is obtained. The blended mixture contains 1800 cm3
C3H6O2, with the assumption that Vmixture = VC3H6O2 + VC3H6O.
[Molecular weight: C3H6O = 58.08; C3H6O2 = 74.08]
- Sketch a completely labelled process flow diagram for the process. marks)
- Estimate molar composition of the blended mixture. (10 marks)
- Estimate the feed flow rates (lbm/s) required to produce 5 lbm/s of blended mixture, if the composition of the product stream remains unchanged. marks)
Q2 An air dehumidifier is used to produce air with water content of 5 mole %. In the process, fresh air containing 18 mole % of water vapor is to be cooled by combining it with a recycle stream of previously dehumidified air. The blended air stream then enters the cooler with 8.5 mole % of H2O. In the air dehumidifier, some water in the blended stream is condensed and removed as liquid. A fraction of the dehumidified air leaving the cooler is recycled while 100 mol of the air is delivered to the chemical plant. Figure Q2 shows the overall flowchart of the cooling and dehumidification process.
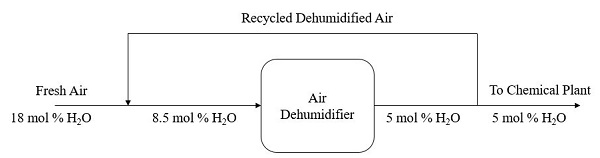
Figure Q2
- Sketch a completely labelled process flow diagram (with compositions) for the process. (5 marks)
- Perform the degree-of-freedom analysis to all possible boundaries for the process. (8 marks)
- Compute the number of moles (mol) of fresh feed, water condensed and dehumidified air recycled. (7 marks)
Q3 (a) Superheated steam at 600°C and 80 bar absolute flows at a rate of 380 kg/min to an adiabatic turbine, where it is expanded to 300°C. If the working capacity or power of the turbine to support the expansion process is 4000 kW, compute the outlet pressure (bar) of the turbine. (10 marks)
(b) A stream of superheated steam (350°C, 60 bar) flowing at 2.5 kg/min is used in a countercurrent adiabatic heat exchanger to heat Isobutane (C4H10) vapour from 130°C to 300°C. The steam condenses and leaves the heat exchanger as liquid water at 64°C. Compute the flow rate of the entering C4H10 vapour in standard liters per minute, L(STP)/min. (10 marks)
Q4 The reaction between hydrogen gas (H2) and carbon monoxide (CO) at high temperature produces methanol (CH3OH) gas. The gaseous feed to the reactor consists of 52 mole % H2, 35 mole % CO, and the remaining is nitrogen (N2). The gaseous feed is flowing at 200 mol/s into the reactor at 120°C, and the product gas emerges from the reactor at 350°C. The limiting reactant is used up in the reaction.
- Compute the molar flow rate (mol/s) of all components in the product stream. marks)
- Compute the amount of heat (kJ/s) that is added or removed from the reactor for the reaction. marks)
Formulas