Octet Rule and Lewis Structure
For a good governance of an association, a set of rules and regulations are required. Similarly, for chemistry, certain rules have been stated to make the study easier and less complex.
As it is said that all matters in this universe have a tendency to attain stability; and to do so the process shall start at the molecular level.
So that’s how it is:
- All the elements discovered till date have an electronic configuration, depicting the number of shells and sub-shells, present in an atom.
- These sub-shells have an upper limit of storing electrons. For example: “S” sub-shell can store 2 electrons, “P” sub-shell can store 6 electrons, “D” sub-shell can store 10 electrons and “F” sub-shell can store 14 electrons.
- Each element in its outermost shell has a capacity of 8 electrons, and to attain the Nearest Noble Gas Configuration the element has to fill this OCTET.
Hence the Octet Rule states that atoms of main-group elements tend to combine in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.
Significance of Octet Rule:
- Octet rule has made it simpler for representation of valence electrons for an atom while boning.
- A number of valence electrons for an atom could be known by octet rule.
- For calculation of oxidation number, the octet rule would be useful.
- Type of bonding and number of electrons participating in bonding could be predicted and known.
Can Octet Rule predict the Stability of an Atom?
Octet rule gives the number of bonding electrons, valence electrons, oxidation number and the number of bonds that would be formed. Hence and the idea of stability could be generated and by knowledge of valence electrons, the stability could be predicted. Yet the stability could not be confirmed as for transition elements, due to the shielding effect of their “d” orbital electrons a steric hindrance is offered which octet rule could not justify. Hence an idea of stability could be generated, but solely one can’t rely on octet rule for the stability of an atom.
Limitations of Octet Rule:
1. The octet rule is not applicable for non-metal after silicon as those elements have a tendency to expand their octet and store more than 8 electrons.
For example:
PF5, SF6, H2SO4.
In PF5, the P atom has 10 electrons in the valence shell; similarly
For SF6 the S atom has 12 electrons in its valence shell.
And for H2SO4 the S atom has again 12 atoms in the valence shell.
2. Atoms with an odd number of electrons do not follow the octet rule. For Example NO and NO2. The N atom in both molecules has 7 electrons in their valence shell. Hence the octet is incomplete.
3. For some molecules, central atom can’t have 8 electrons. For example BeCl2 and BCl3. Be has only 4 electrons in valence shell and B has 6 electrons. Hence the octet rule is not valid in such cases.
Hence the OCTET rule has been a great rule for determining the valence electrons and determining the number of bonding electrons in a molecule.
Lewis Dot Structure:
Through octet rule we determined the number of valence electrons and bonding electrons, now to see how bonding happens a sharing of electrons take place we need to draw some basic structures. One of the structures is the Lewis Dot Structure.
What is a Lewis Dot Structure?
The diagrammatical representation of bonds being formed between valence shell-electrons and the lone pair electrons present in an atom is termed as Lewis Dot Structure.
The significance of Lewis Dot Structure:
- This structure shows the bonding electrons and number of bonds being formed.
- The structures give the number of electrons left out of bonding, that would generate a negative charge on the molecule.
- The concept of RESONANCE has generated and is justified by these structures.
- Nuclear stability has also been proven by Lewis Dot Structures.
- The concept of Formal Charge got introduced.
How to Draw Lewis Dot Structures?
- Write the electronic configuration of the elements involved in bonding.
- Determine the valence electrons for each element.
- Write the symbol for each element followed by dots over the symbol, representing the number of valence electrons.
- Now start connecting the valence electrons of the first element to the valence electrons of the second element.
NOTE- one electron is shared with only one electron of the other element and as the number of electrons being shared with each other reaches 8 stop connecting the dots and the Lewis Dot Structure is Ready.
Example:
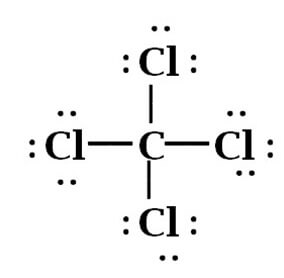
From the above-mentioned Lewis Structure of CCl4, it is Clearly depicted that Carbon being tetravalent has made 4 covalent bonds with Chlorine, where each Chlorine Atom has shared one electron with each Carbon electron.
(http://weknownyourdreamz.com/symbols/chlorine-electron-dot-symbol.html) refer the link for more images.
The concept of Formal Charge:
As in Lewis structure, the number of bonding and non-bonding electrons could clearly be determined. Now the electrons that don’t take part in the bonding would induce charges on the molecule formed, yet the molecule remains stable and electrically neutral. This stability is explained by the concept of Formal Charge.
Hence formal Charge is defined as the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity.
Formula for Formal Charge:
FC = V - N - (B/2)
Where
- FC -> formal charge
- V -> number of valence electrons of all the elements participating in bonding
- N -> number of non-bonding electrons
- B -> number of bonding electrons
Benefits of Lewis Dot Structures:
- Using Lewis Dot Structure, the number of bonding and non-bonding electrons could be determined.
- A number of covalent bonds and hence the strength of the bond could be predicted.
- Formal charge and charge distribution on an atom could be determined.
Hence, with the Lewis Dot Structure, the nature of a bind could easily be predicted and further calculations involving electrons could also be carried out.


