Water pump efficiency used lift water upto the overhead tank
|
1 |
|---|
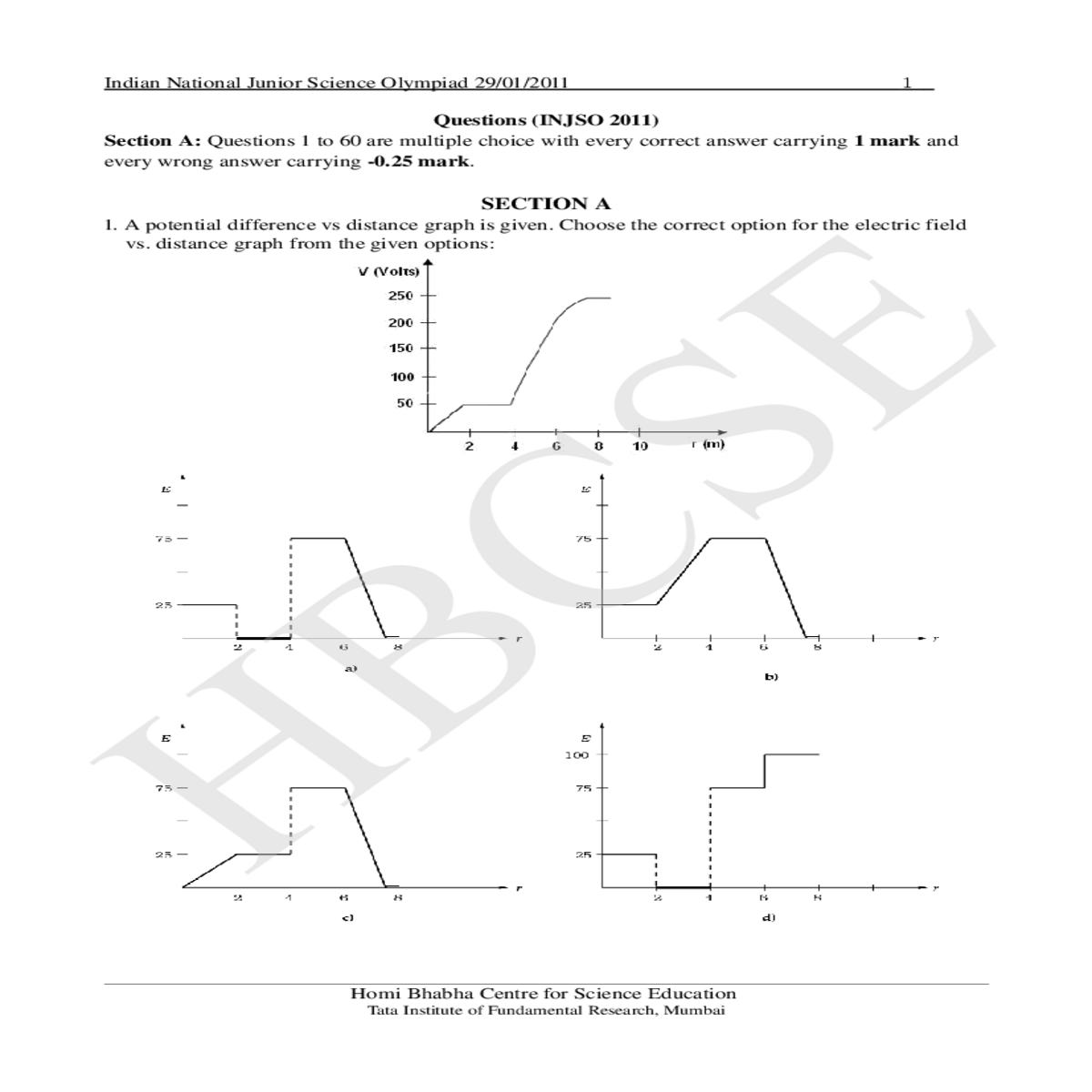
Questions (INJSO 2011)
Section A: Questions 1 to 60 are multiple choice with every correct answer carrying 1 mark and every wrong answer carrying 0.25 mark.
|
2 |
|---|
2. Separate solutions of HCl (aq) and H2SO4 of the same molar concentration and same volume were completely neutralized by NaOH (aq). X KJ and Y KJ of heat were evolved respectively. Which statement is correct?
a) Menstrual phase
b) Proliferative phase
c) Ovulatory phase
d) Luteal or Secretory phase
4. Muscles containing large amounts of Myoglobin are likely to be found in a...
| a) 2 | b) 3 | c) 4 | d) 5 |
| 3 |
|---|
6. A ball is dropped from a height h on a floor and suffers multiple perfectly elastic bounces. The velocity vs t graph is as shown. Here time T depicts the time required to complete one cycle.

|
c) ferrimagnetic |
|
|---|
| 4 |
|---|
| b) H – h | c) H.h | d) H /h |
|---|
9. Anil, an 8th grade student, was asked to draw a figure explaining how the mammalian eye collects and focuses light, converting it into electrical signals. Which of the following flow charts correctly represents the process?
a) LightCorneaAqueous humorPupilLensVitreous humorRetina Action potentials in neuronsOptic nerveBrain.
| a) 4 HP | b) 240 HP | c) 3.2 HP | d) 2.4 HP |
|---|
11. When a compressed gas is allowed to expand through a small orifice cooling effect is caused if
a) the temperature of the gas is less than the inversion temperature (Ti) b) the temperature of the gas is greater than the inversion temperature ( Ti) c) the temperature of the gas is equal to the critical temperature
d) the temperature of the gas is 273K.
| 5 |
|---|
13. A student adds 5.85 gm of NaCl to 1 litre of water (the pH of which was measured to be 7.0) in a flask (X) to make a 0.1 M solution. He transfers 500 ml into another flask (Y). He covers the flask (Y) with tissue paper and the original flask (X) with a watch glass and goes to watch a movie. When he returns to the lab the next morning, he checks the pH of both the solutions using a perfectly calibrated pH meter. Which of the following is correct?
a) X has pH = 7 and Y has pH > 7
b) X has pH < 7 and Y has pH = 7
c) X has pH = 7 and Y has pH < 7
d) Both X and Y have pH = 7a) only i
b) only ii
c) i and ii
d) i, ii and iii16. Two species live in the same locale but each one reproduces at different time of the year & both do not attempt to mate each other. This can be considered as an example of:
| 6 |
|---|
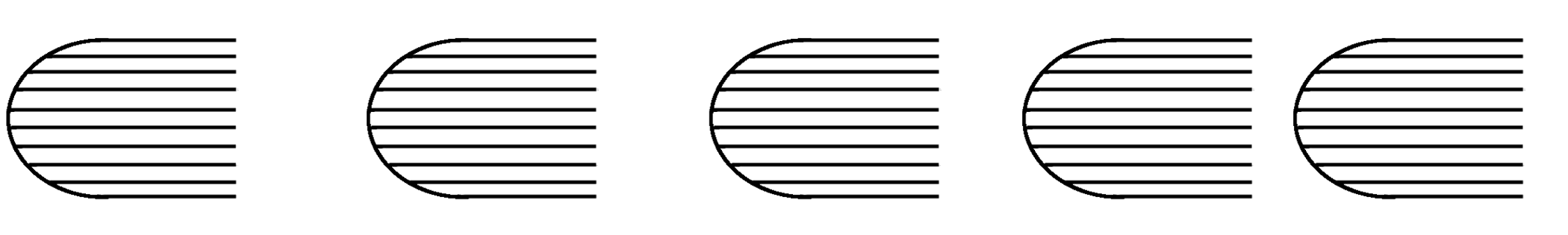
18. A vehicle is moving on a road. Ink drops are falling, one at a time, on the road from the vehicle. After the vehicle has moved away, what one observes is shown (qualitatively) in the figure given below. From the figure we can conclude about the vehicle to be moving...
| b) ¼W | c) W | d) 4W |
|---|
|
b) Custard apple, Fig c) Pineapple, Jackfruit |
|
|---|
frictional force acting on the block in N are respectively
| a) 0, 20 |
|
c) 30, 25 | d) 0, 15 |
|---|
| 7 |
|---|
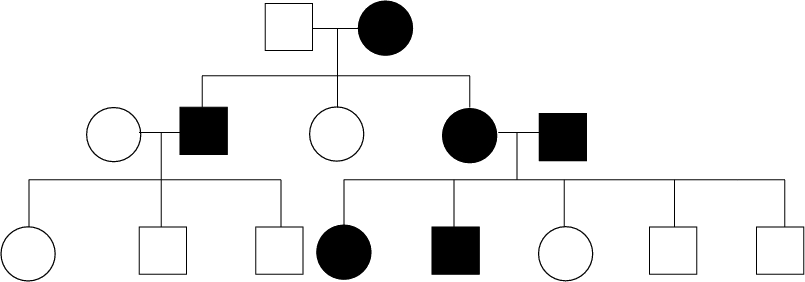
24. In the following cross, the character indicated by males (darkened squares) and females (circle) is...
|
|
|---|
28. What will be the volume of Cl2 at STP produced during electrolysis of MgCl2 which produces 6.5g Mg (At.wt. of Mg = 24.3g, Cl = 35.5g)
a) 5.099 litre b) 5.99 litre c) 12.02 litre d) 3.099 litre
| 8 |
|---|
| a) 6Ω | b) 2.5Ω |
|
|---|
30. It is known that among corn plants, a tall plant (T) trait is dominant over dwarf (t), and the coloured kernel (C) trait is dominant is over white (c). Which of the following results represents the outcome of a cross between contrasting dihybrid parents?
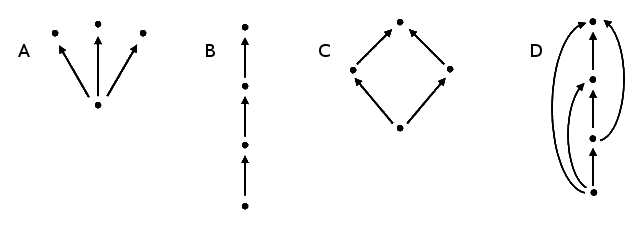
| a) A | b) B | c) C | d) D |
|---|
Homi Bhabha Centre for Science Education
Tata Institute of Fundamental Research, Mumbai
| 9 |
|---|
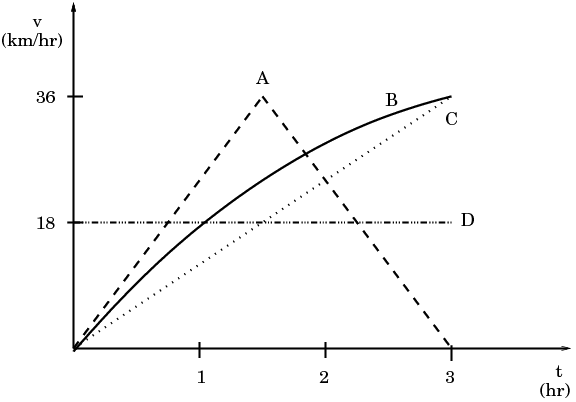
| a) A | b) B | c) C | d) D |
|---|
35. A certain sample of concentrated hydrochloric acid contains 50% HCl by mass and has density 1.20 gcm3. What is the molarity of this sample?
| 10 |
|---|
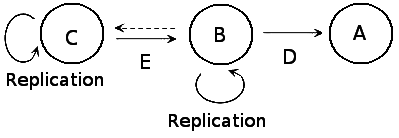
a) A = Protein, B = RNA, C = DNA, D = Translation, E = Transcription b) A = RNA, B = DNA, C = Protein, E = Transcription, D = Translation c) A = Translation, B = DNA, C = Protein, D = Transcription, E = RNA d) A = DNA, B = RNA, C = Protein, D = Transcription, E = Translation
3rd mirror. The ray after reflection from 3rd mirror will be
a) passing through the same point.
i) BaO2 (s) + O3 (g) → BaO (s) + 2O2 (g)
ii) ZnS (s) + 4O3 (g) → ZnSO4 (s) + 4O2 (g)
| 11 |
|---|
a) Forming Silicon complex with clay particles
b) Sulphate part which combines with dirt and removes it
c) Compound of Aluminium which coagulates the mud particles.d) Making mud water soluble
|
|||
|---|---|---|---|
|
190 cm | 190 cm | 180cm |
| 60 Kg | 65Kg | 75 Kg | |
| O | AB | O | |
| Measure of intelligence | 135 | 140 | 125 |
| White | White | Dark |
| a) AB | b) AC | c) CB | d) BC |
|---|
combination across a source of emf having stabilized voltage and negligible resistance, all bulbs
glow with full brightness. Suddenly a bulb fuses. The other bulbs will glow..
|
12 |
|---|
44. Rust is a mixture of
a) FeO and Fe(OH)3
b) FeO and Fe3O4
c) Fe2O3 and Fe(OH)3
d) Fe(OH)3 and Fe3O4
| Sr |
|
|
|||
|---|---|---|---|---|---|
| 1. | Yes | Yes | No | No | |
| 2. | No | Yes | Yes | Yes | |
| No | Yes | Yes | No | ||
| 4. |
|
No | No | Yes | No |
a) Plant group I
b) Plant group II
c) Plant group III
d) Plant group IVHomi Bhabha Centre for Science Education
Tata Institute of Fundamental Research, Mumbai
|
13 |
|---|
|
|---|
a) A)(ii); B)(iv); C)(i); D)(iii)
b) A)(ii); B)(iii); C)(iv); D)(i)
c) A)(i); B)(iv); C)(ii); D)(iii)
d) A)(iv); B)(ii); C)(i); D)(iii)
| i) h2h3 | ii) h1h2 | iii) A2/A1 |
|
14 |
|---|
51. In the Haber process for the synthesis of ammonia, amount of ammonia formed will be more if: a) pressure is decreased and temperature is increased
b) pressure is increased and temperature is decreased
c) both pressure and temperature are increased
d) both pressure and temperature are decreased
|
A) Naturally acquired ACTIVE IMMUNITY |
|
|
|---|---|---|---|
| B) Artificially acquired ACTIVE IMMUNITY | |||
|
C) Naturally acquired PASSIVE IMMUNITY |
|
|
| D) Artificially acquired PASSIVE IMMUNITY | iv) Ria was advised by doctors to breast feed her new born in order to improve infant's immunity. |
Homi Bhabha Centre for Science Education
Tata Institute of Fundamental Research, Mumbai
|
15 |
|---|
a) i, ii, iii,v, vi
b) ii, iv and vii
c) ii, iii, iv, vii,viii
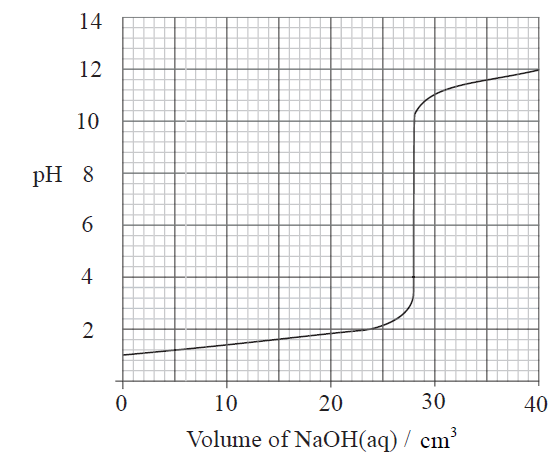
d) i, iii, v,vi, viii58.The pH of solution Xis 2 and that of Yis 4. Which statement is correct about the hydrogen ion concentrations in the two solutions?
|
16 |
|---|
59. In the figure given below what is the value of R between points A and B?
| a) 2R 3 | b) R | c) 0 | d) R 2 |
|---|
SECTION B (Long questions)
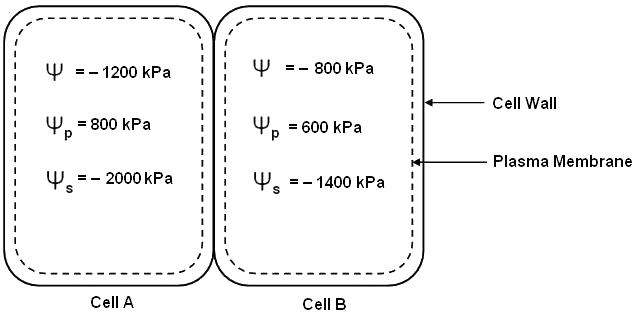
61. Osmosis is the movement of water molecules from a region of their higher concentration (dilute solution) to a region of their lower concentration (concentrated solution) through a semipermeable membrane. Water potential is the tendency of water molecules to move from one place to another through membranes. It is denoted by ψ and is measured in terms of the unit called “pascals”.
| 17 |
|---|
ψ = ψs + ψp
Using the above description, answer the following questions.
3. Following are two neighboring plant cells in contact with each other.
Homi Bhabha Centre for Science Education
Tata Institute of Fundamental Research, Mumbai
|
18 |
|---|
|
|||
|---|---|---|---|
|
(1.5 Marks) | ||
| (1 Mark) | |||
| (0.5 Mark) | |||
|
|||
65. A rise in the ocean level is expected on account of the melting of icebergs due to global warming. The iceberg R15 broke off the Ross IceShelf in Antarctica and plunged into the ocean in 2000. We estimate the rise in ocean level due to this event. The iceberg was made of fresh water and shaped as a cuboid of cross sectional area A = 10000 km2 and height h = 0.4 km. The total ocean surface area is 3.61 × 108 km2 and ocean water has density ρo = 1024 kg∙m−3 .
i. What is the rise in the ocean level due to plunging of iceberg in the ocean?
|
19 |
|---|
66.a) Prove that a square of a natural number leaves either 0 or 1 as remainder upon division by 4.
(2 Marks)
Homi Bhabha Centre for Science Education
Tata Institute of Fundamental Research, Mumbai
|
20 |
|---|
2. Name the enzymes from the following list of enzymes which could representa) activity curve A
b) activity curve BEnzymes: Chymotrypsin, Pepsin, Sucrase, Salivary amylase, Pancreatic lipase, Catalase.
| pH of solution | Time to collect gas/min |
|---|---|
| 4 | 20 |
| 5 | 12.5 |
| 6 | 10 |
| 7 | 13.6 |
| 8 | 17.4 |