|
Platelets |
Degranulation |
Xa |
| GP |
|
|
| IIb/IIIa |
Activation
|
| GP |
Fibrin |
+ |
| IIb/IIIa |
| Thrombin |
Prothrombin
|
|
|
and edema and is tender to touch. Oxygen saturation by
|
ment complaining of acute onset of shortness of breath and
|
fingertip pulse oximeter while breathing room air is
87% |
|
(normal > 90%). Ultrasound reveals a deep vein
thrombosis |
|
in the left lower extremity; chest computed tomography
scan |
|
confirms the presence of pulmonary emboli.
Laboratory |
|
blood tests indicate elevated d-dimer levels. What therapy
|
aggregation and vasoconstriction. Activation of platelets results
in
|
|
|
is indicated acutely? What are the long-term
therapy |
|
options? How long should she be treated? Should this
indi- |
|
vidual use oral contraceptives? |
a conformational change in the αIIbβIII integrin (IIb/IIIa)
recep-
|
This occurs when part or all of the clot breaks off from its
location
|
|
|
|
|
| Hemostasis refers to the finely
regulated dynamic process of main-taining fluidity of the blood,
repairing vascular injury, and limit-ing blood loss while avoiding
vessel occlusion (thrombosis) and |
MECHANISMS OF BLOOD
COAGULATION
|
(Figure 34–1). Simultaneously, the coagulation system cascade
|
Occlusion of a large pulmonary artery by an embolic clot
can pre- |
|
|
|
|
hemostatic mechanism is important for diagnosis of bleeding
|
arterial segment. Such emboli usually arise from the deep venous
|
|
|
| inadequate perfusion of vital organs.
Either extreme—excessive bleeding or thrombosis—represents a breakdown
of the hemo-static mechanism. Common causes of dysregulated hemostasis
include hereditary or acquired defects in the clotting mechanism and
secondary effects of infection or cancer. Atrial fibrillation is
associated with stasis of blood in the atria, formation of clots, and
increased risk of occlusive stroke. Because of the high prevalence of
chronic atrial fibrillation, especially in the older population, use of
anticoagulants is common. Guidelines for the use of oral anticoagulants
(CHA2DS2-VASC score, see January C et al refer-ence)
are based on various risk factors (congestive heart
failure, hypertension, age,
diabetes, history of stroke,
vascular disease, and sex). The drugs used to inhibit
thrombosis and to limit abnor-mal bleeding are the subjects of this
chapter. |
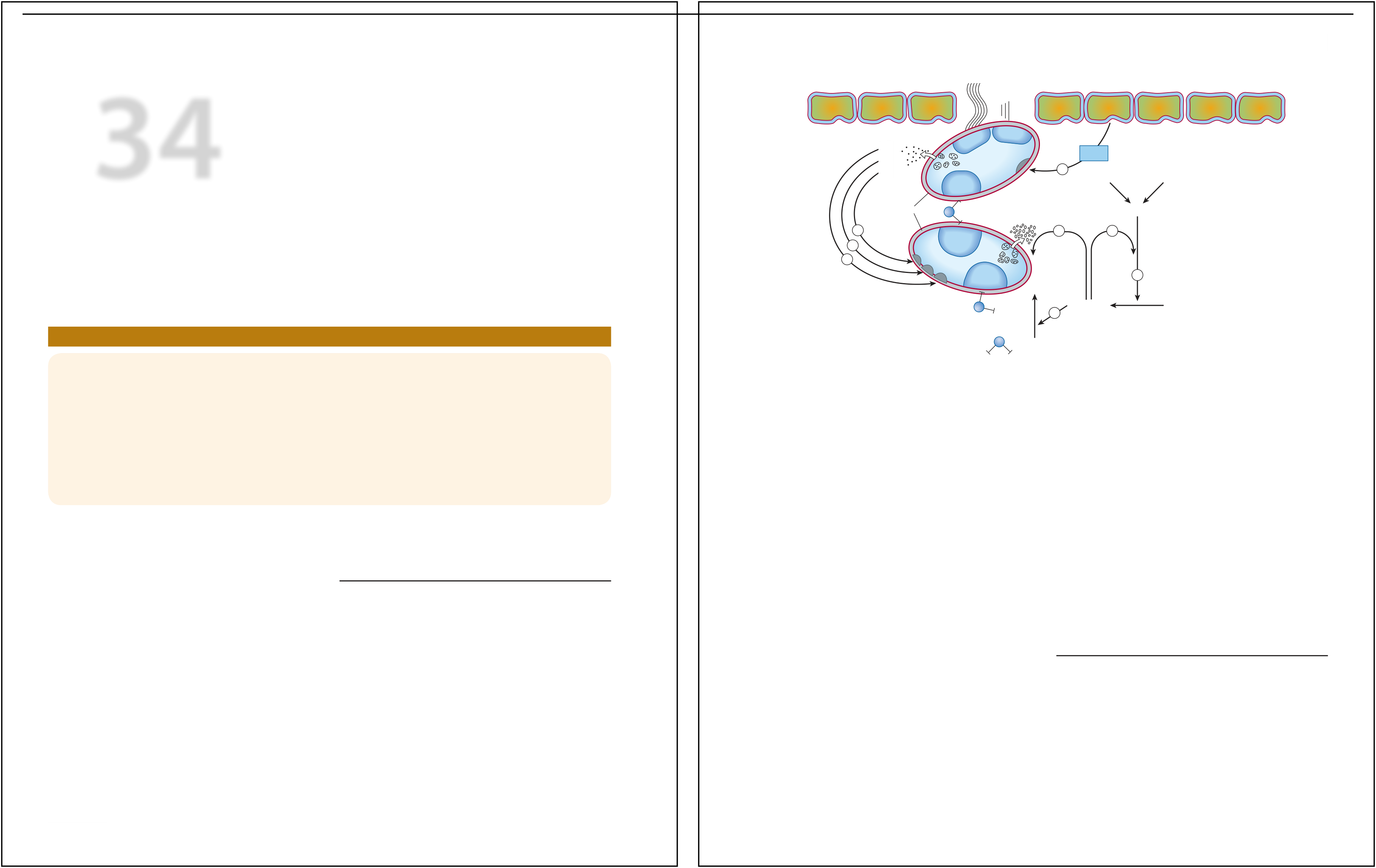
The vascular endothelial cell layer lining blood
vessels has an anticoagulant phenotype, and circulating blood platelets
and clotting factors do not normally adhere to it to an appreciable
extent. In the setting of vascular injury, the endothelial cell layer
rapidly undergoes a series of changes resulting in a more proco-agulant
phenotype. Injury exposes reactive subendothelial matrix proteins such
as collagen and von Willebrand factor, which results in platelet
adherence and activation, and secretion and synthesis of
vasoconstrictors and platelet-recruiting and activating molecules. Thus,
thromboxane A2 (TXA2) is synthesized from arachidonic
acid within platelets and is a platelet activator and potent
vasoconstrictor. Products secreted from platelet granules include
adenosine diphosphate (ADP), a powerful inducer of
platelet aggregation, and serotonin (5-HT), which
stimulates |
|
thrombi are mixed, the platelet nidus dominates the
arterial throm- |
|
bus and the fibrin tail dominates the venous thrombus.
|
(gingiva, skin, heavy menses) with injury. In contrast,
patients
with defects in the clotting mechanism (secondary hemostasis,
|
|
|
|
|
Blood coagulates due to the transformation of soluble fibrinogen
|
| The platelet is central to normal hemostasis and
thromboem- |
into insoluble fibrin by the enzyme thrombin. Several
circulat- |
bolic disease, and is the target of many therapies discussed in
this
|
|
|
|
|
undergoes limited proteolysis and becomes an active protease
|
arterial thrombi cause serious disease by producing downstream
|
|
|
|
|
the formation of thrombin (factor IIa). Several of these factors
are
|
rich, contain large numbers of trapped red blood cells, and are
|
|
|
|
|
tions. In clotting, thrombin proteolytically cleaves small
peptides
|