Sums protons and neutrons the nuclei that must balanced
Nuclear Fission & Fusion:
Nuclear Reactions:Chemical reactions only involve the rearrangement of valence electrons and the changing of bonding patterns of atoms. Although this may seem to be a statement of the obvious, atoms do not change their identities and mass is conserved during chemical reactions. The energy changes of chemical reactions are relatively small. Nuclear reactions, on the other hand, involve rearrangements of the nuclei involved in the reactions which actually does result in atoms changing their identities.
(note: 238 = 234 + 4 and 92 = 90 + 2)
It is important to note that nuclear reactions only involve nuclei. This is why states are not shown in the equations; the states are in fact irrelevant. The uranium nucleus being described in the above example may not even be in a free uranium atom. Even if the uranium atom was part of a UF6 molecule, the nuclear reaction would be the same. Surrounding environmental conditions and chemical environments do not affect nuclearreactions except sometimes under extreme circumstances (e.g. millions of °C).
| 1 | ! |
||
|---|---|---|---|
| 1 | |||
|
1 | ||
| 0 |
1 or β !
|
He | ||
|---|---|---|---|
| gamma-ray (a high energy photon) | |||
Here is an example involving the bombardment of a nucleus with a high energy particle:
4 9Be+ 2 4He → 6 12C + 0 1n
(b) beta-decay: 239Np → ? ? ?+ −1 0e
(c) positron-decay: ? ? → 14 30Si+ 1 0e
(b) 𝟐𝟑𝟗 𝟗𝟒 𝐏𝐮 because 93 = 94 + (-1), 239 = 239 + 0, and Pu is element #94
(c) 𝟑𝟎 because 15 = 14 + 1, 30 = 30 + 0, and P is element #15 𝟏𝟓𝐏
Energies of Nuclear Reactions:
The energy changes involved in nuclear reactions are very large; as you will see, this is a vast understatement. The chart below shows typical energy changes associated with different reaction types in kJ of energy released per gram of reactant.
| example reaction | type of reaction | energy released / kJ g-1 |
|---|---|---|
| condensation of H2O (steam) | physical change | 2 |
| complete combustion of CH4 | chemical reaction | 56 |
| induced fission of 235U | nuclear fission | 88 000 000 |
| fusion of 2H and 3H | nuclear fusion | 340 000 000 |
| 228 90 Th | |
|
+ |
|
|---|
| note: kg m2 s-2 = J |
|---|
This seems like a very small energy, but consider that it is for only one atom of 228Th. For a mole, the energy is greater by a factor of 6.022×1023.
! |
|
|---|
And to get a number comparable to the chart on the previous page, convert to per gram.
| !" | ||
|---|---|---|
| !"# |
Nuclear binding energy per nucleon is the energy absorbed per nucleon during the theoretical separation of a nucleus into its free nucleons. It is a measure of nuclear stability. Consider the following related definitions:
mass defect = Δm = (sum of masses of individual nucleons) - (mass of nucleus)
nuclear binding energy
number of nucleons in nucleusNuclear binding energy per nucleon can be used to compare the relative stability of nuclei and to predict the type of nuclear reactions that a nucleus will undergo.
Notes regarding the graph:
• Mass number = number of nucleons in the nucleus.• MeV (i.e. Megaelectron volt) is a unit of energy.
• Both fusion and fission can lead to increased nuclear stability.
• The fact that the increase in nuclear binding energy per nucleon for very small nuclei undergoing fusion is much greater than the increase in nuclear binding energy per nucleon for large nuclei undergoing fission is why fusion releases far more energy per gram of reactant that fission.
| 235 92 U | + | 1 | n | →[ | 236 | U |
56 Ba |
+ | 92 | Kr | + ⋅3 | 1 | n | + | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 92 | 36 | 0 |
It is possible for the neutrons produced by the example reaction to induce more uranium nuclei to undergo fission. Critical mass is the mass of fissionable material, in this case 235U, in a certain volume and geometry needed to sustain a chain reaction. A chain reaction occurs when the uranium sample is large and dense enough so that 1 of the neutrons produced from each reacting nucleus causes another nucleus to react. The other 2 neutrons escape or react with something other than 235U. For a given volume and geometry, a subcritical mass is any mass that is smaller than the critical mass and can not sustain a chain reaction. A supercritical mass is any mass that is larger than the critical mass in which case more than 1 of the neutrons from each fissioning 235U atom are captured by other 235U atoms. Supercritical masses are needed for nuclear weapons and result in uncontrolled chain reactions whose rates accelerate exponentially.
Sample Question #2: Write the balanced nuclear reaction equation for the neutron induced fission of 235U. The products are 139Ba, three neutrons, and one other nucleus.
| U | + n !! | !"# ? Ba |
|---|
The other product must be element #36 (92 + 0 = 56 + 36 + 3×0) which is Kr. The mass number for Kr must be 94 (235 + 1 = 139 + 94 + 3×1).
| ∴ |
|
𝐔 | | 𝟏𝟑𝟗 𝟓𝟔 𝐁𝐚 | + | 𝟗𝟒 𝟑𝟔𝐊𝐫 |
|---|
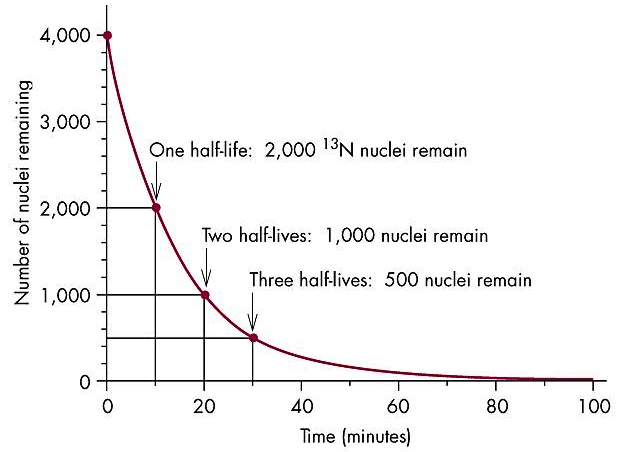
Half-Life:
| 0 | 100 | (½)0 = 1 |
| 1 | 50 | (½)1 = 1/2 |
| 2 | 25 | (½)2 = 1/4 |
| 3 | 12.5 | (½)3 = 1/8 |
| 4 | 6.25 | (½)4 = 1/16 |
| 5 | 3.125 | (½)5 = 1/32 |
Number of nuclei remaining vs. time elapsed
Note: From 7.11 Isotope half-life, 1998, McGraw-Hill Higher Education.
75.0 cpm = 0.125 = (½)3 ∴ 3.00 half-lives has elapsed |
|---|
| U |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| !"# !" U | | Np | + |
|
||||||||
| !"# !" Np | | !"# !" Pu | + | ! |
||||||||
Advantages:
• The fuel is technically nonrenewable, but the fuel is quite plentiful.• If nuclear fission reactors operate properly and there are no catastrophic events, then there are only limited problems associated with there use.
• The actual health risks from radioactive material include cancers, birth defects, and mutations. With major exposure, organ damage is possible in a short time-frame.
Nuclear Waste:
Nuclear Fusion:
Nuclear fusion involves two light nuclei fusing together to form a heavier nucleus.
| 1 | H | + | 2 | H | → | 3 | He | + | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 |
| ! !H | + H ! ! |
|---|
The advantages of fusion if it could be made to work are:
• Fuel is extremely abundant, relatively cheap to process, and widely distributed. • Fusion releases enormous quantities of energy per kg of fuel.• Fusion does not produce radioactive waste as does fission.
• Enough net energy production must occur to make the process economically feasible.
________________________________________________________________________
Note: From Quantum Mechanics Timeline, Ideas of Physics Homepage, 2012, David Taylor.
Since the emission spectra of all elements are known and since elements absorb the same colours that they emit when excited, the dark spectral lines in the solar spectrum can be attributed to particular elements proving their existence in the sun. Helium was originally “discovered” as a result of the appearance of spectral lines in the solar spectrum that
A. Nuclear reactions are reactions of the nucleus.
B. Nuclear reactions are typically affected by environmental conditions.
#4. |
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. | ||||||||||||||
| B. | ||||||||||||||
| C. | ||||||||||||||
| D. | ||||||||||||||
|
||||||||||||||
| (a) |
|
|||||||||||||
| (b) | Xe | + _____ | ||||||||||||
| (c) | U | + n !! |
|
+ | !"Sr !" |
|
||||||||
| (d) | !"# !" Tl | |||||||||||||
| (e) | ! !H | ! |
|
|||||||||||
| (f) | ||||||||||||||
| B. |
|
|||||||||||||
constituent protons and neutrons.
|
|||||
|---|---|---|---|---|---|
|
U | + n !! | !"# !! Cs | ||
| For a particular radioactive isotope, the graph of radiation detected (in counts per | |||||
Note: From activity and half-life of a radioactive source, GCSE Physics, n.d., Frank’s Web Space.
| #7. |
|
|---|
B. The vast majority of nuclear power reactors world-wide are based on fusion.
C. The centre of the sun has a temperature of 15 million K due mainly to the heat released from fusion reactions.
and the fast decay of the isotopes is why the waste is so radioactive.
B. High level nuclear waste mainly comes from the nuclear power industry
in geologically stable abandoned mine sites thus isolating it from people
and the environment.
D. Fusion releases the most energy per kg of fuel of any known process.
#13. If fusion ever becomes technologically and economically feasible as a source of
#14. #15. |
||
|---|---|---|
| (d) |
|
|
|
||





