Slip occurs the most densely packed crystallographic planes and
| N | M |
|
● | S-5 | |
|---|---|---|---|---|---|
| N | M | ||||
 |
|||||
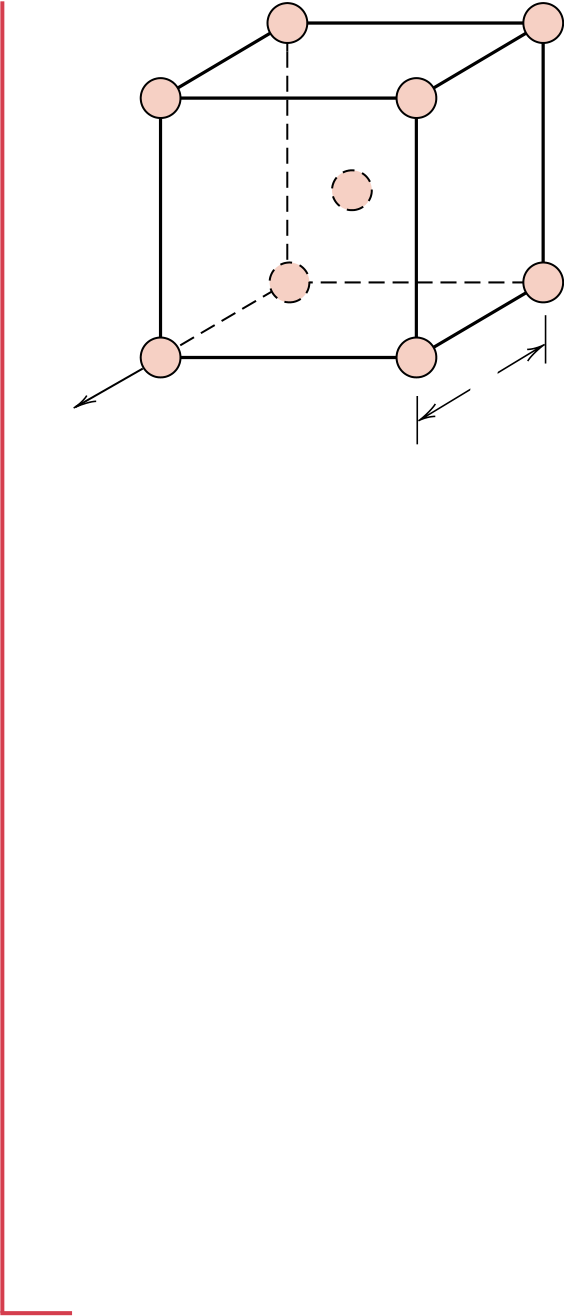
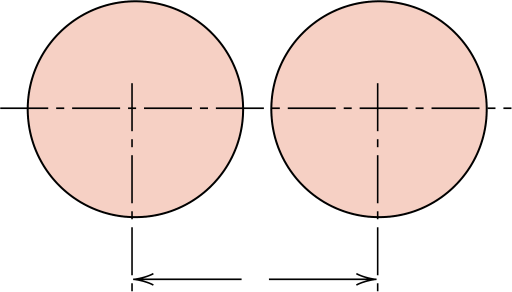
As a basis for our computation let us use the line length within the unit cell, Ll, which in this case is the lattice parameter a—the distance between the centers of atoms M and N. In terms of the atomic radius R,
|
(see Equation 3.3) |
|---|
| LDLc | 2R | ||||
|---|---|---|---|---|---|
|
� | 4R/�3 |
Calculate the planar density of the (110) plane for FCC.
S O L U T I O N
Therefore,
Ap � (AC)(AD)
| S-6 | ● | |
|---|---|---|
PD �Ac |
Historically much of our understanding regarding the atomic and molecular arrange-ments in solids has resulted from x-ray diffraction investigations; furthermore, x-rays are still very important in developing new materials. A brief overview of the diffrac-tion phenomenon and how, using x-rays, atomic interplanar distances and crystal structures are deduced will now be given.
THE DIFFRACTION PHENOMENON
X-rays are a form of electromagnetic radiation that have high energies and short wavelengths—wavelengths on the order of the atomic spacings for solids. When a





