Positively charged centre now called the nucleus
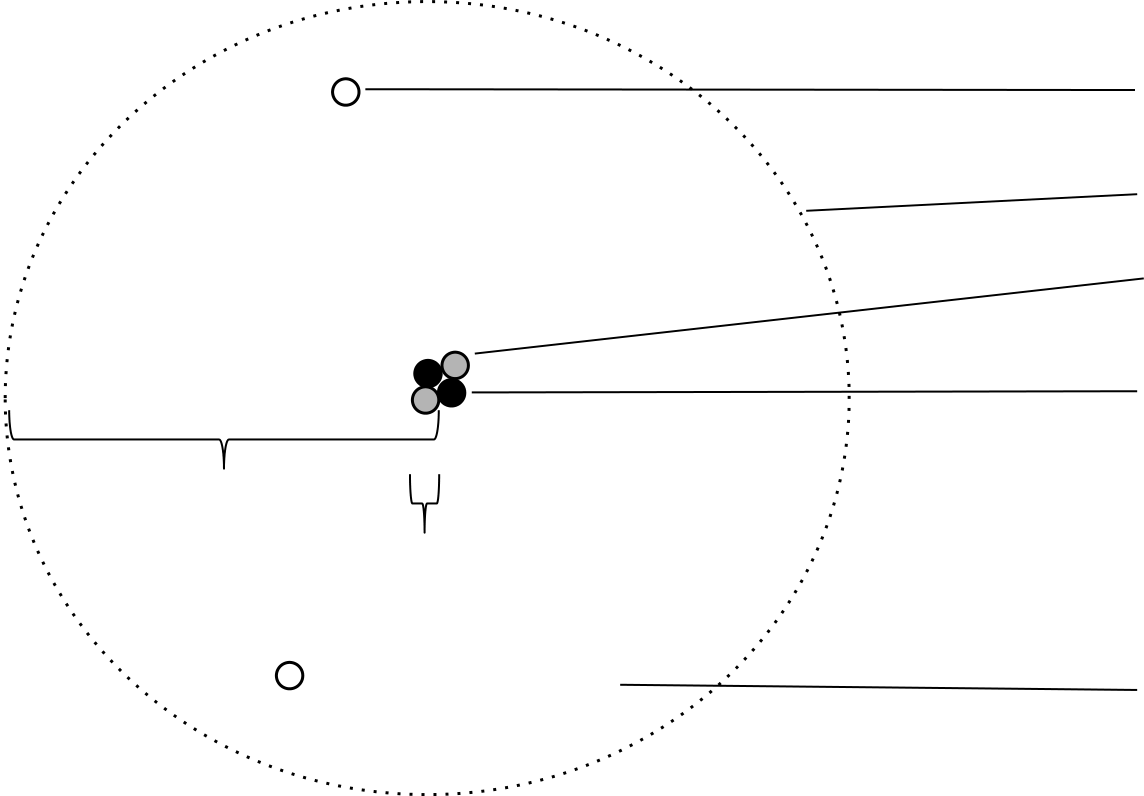
The Nuclear Model of the Atom:
| particle | relative charge | precise mass / kg | approximate relative mass |
|---|---|---|---|
| proton | +1 | 1.672 62 × 10-27 | 1 |
| neutron | 0 | 1.674 93 × 10-27 | 1 |
| electron | -1 | 9.109 39 × 10-31 | 1 / 1837 |
The idea of sub-atomic particles introduces a major challenge to Dalton’s theory. Scientists have chosen to add to the theories of matter to accommodate this more complex understanding instead of entirely replacing the old theories that are still useful in many respects. Subatomic particles and the nuclear model constitute a paradigm shift in how people thought about matter.
The Nuclear Atom: There were several theories regarding how electrons fit into the atom (e.g. the plum pudding model) most of which were incorrect. Experimental evidence eventually led to the theory that most of the mass of an atom is concentrated in a very small, dense, positively charged centre now called the nucleus. It is now known that protons and neutrons are located in the nucleus of the atom. Protons and neutrons are collectively known as nucleons. Most of the remainder of the atom is empty space throughout which the electrons are located. Since protons and neutrons are much heavier than electrons, almost all of the atom’s mass is located within a tiny fraction of the atom’s volume. In fact, atomic radii range from about 0.03 nm to 0.3 nm while nuclear radii range from 1.2 × 10-6 nm to 7.5 × 10-6 nm. This means that the nucleus occupies less than one trillionth of the volume of the atom.
10-1 nm
10-6 nm
e.g. carbon has 6 protons and 6 electrons
lead has 82 protons and 82 electronsIsotopes are atoms of the same element that contain different numbers of neutrons and therefore have different masses. Note that protons and neutrons both have relative masses of about 1, and electrons have negligible relative mass when rounding to the nearest unit.
e.g. compare 19 40K to Z AX
# of protons = Z = 19
# of neutrons = A - Z = 40 - 19 = 21
# of electrons = # of protons = Z = 19
A radioisotope has an unstable nucleus which results in its nucleus breaking down to form a different, but more stable, nucleus. This constitutes a nuclear reaction, not a chemical reaction. The nuclear breakdown is accompanied by the emission of radiation. This radiation carries with it large amounts of energy and can be of different forms: fast moving alpha or beta particles, and/or gamma-rays which are themselves a form of energy. Radioisotopes emit varying energies at varying rates depending on the isotopes involved. The exposure of living tissues to radiation can have negative effects
(e.g. burns, cancer, and cataracts). Genetic damage to reproductive cells can cause mutations and birth deformities.The Uses of Radioisotopes: Radioisotopes have many uses in the modern world.
| (c) |
|---|
Ions: Atoms and molecules are by definition neutral and thus have equal numbers of
| electrons and protons. Ions have charges. Charge is indicated in symbols with a number | |
|---|---|
|
|
|
|
|---|---|
|
|
|
|
|---|
| e.g. | !" !" | 𝑨 𝒁 | 𝑿𝒒 | |
|---|---|---|---|---|
| 𝑁𝑖!! | ||||
| e.g. | !" !" |
|
|---|
# of protons = Z = 34
Nuclear Model Review Questions: Select the one correct response in each question.
#1. |
|
|
|---|---|---|
|
||
|
||
|
||
|
|
|
|
||
|
||
|
||
|
||
|
|
|
|
||
identity of the element?
| A. | |||
|---|---|---|---|
| B. | |||
| C. |
|
||
| D. |
|
||
| A. | |||
| B. | |||
| C. | |||
| D. |
|
||
|
|||





