Papaya mealybug and madeira mealybug
Okra from India: biosecurity import requirements final report
March 2023
© Commonwealth of Australia 2023
Inquiries about the licence and any use of this document should be emailed to copyright@aff.gov.au.
GPO Box 858 Canberra ACT 2601
Telephone 1800 900 090
Acknowledgement of Country
We acknowledge the Traditional Custodians of Australia and their continuing connection to land and sea, waters, environment and community. We pay our respects to the Traditional Custodians of the lands we live and work on, their culture, and their Elders past and present.
1.2 This risk analysis 1
2 Commercial production practices for okra in India 7
2.5 Harvesting and handling procedures 15
2.6 Post-harvest 16
3.3 Overview of pest risk assessment 25
3.4 Peach fruit fly and melon fly 27
3.9 Pest risk assessment conclusions 42
4 Pest risk management 45
4.5 Meeting Australia’s food laws 53
5 Conclusion 55
References 184
Figures
Figure 2.3 Typical okra crop 13
Figure 2.4 Okra being harvested 16
Figure 3.1 Overview of the PRA decision process for okra from India 44
Figure A.1 Decision rules for determining the impact score for each direct and indirect criterion, based on the level of impact and the magnitude of impact 64
Table 2.4 Peak okra growing periods in major okra producing states 23
Table 3.1 Quarantine pests and regulated articles associated with okra from India, and requiring further pest risk assessment 25
Table 3.6 Risk estimates for quarantine thrips 35
Table 3.7 Risk estimates for emerging quarantine orthotospoviruses vectored by regulated thrips 35
Table A.3 Decision rules for determining the overall consequence rating for each pest 65
Table A.4 Risk estimation matrix 65
Map 4 Top 10 production states of okra in India 2017 to 2018 8
Map 1 Map of Australia
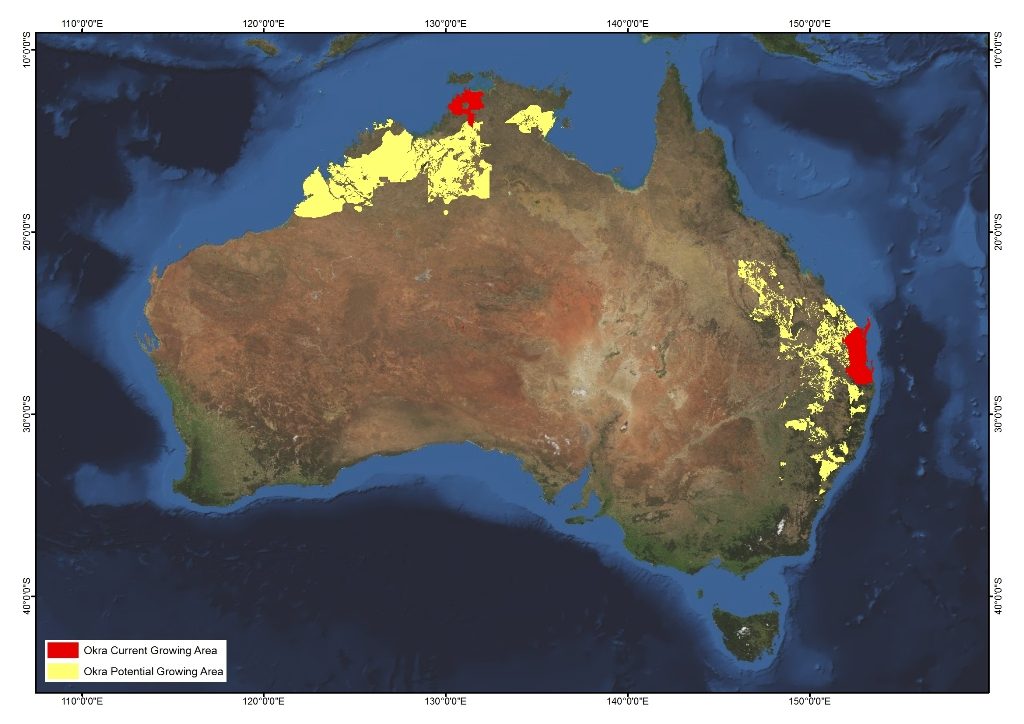
Source: AgriFutures Australia (2017)
Summary
Nine quarantine pests have been identified in this risk analysis as requiring risk management measures to reduce the biosecurity risk to an acceptable level. These pests are:
fruit flies: peach fruit fly (Bactrocera zonata) and melon fly (Zeugodacus cucurbitae)
The 2 quarantine thrips were also assessed as regulated articles for all of Australia, as they are capable of harbouring and spreading emerging orthotospoviruses that are quarantine pests for Australia.
An additional species, chilli thrips (Scirtothrips dorsalis), has been assessed as a regulated article for Australia as it is capable of harbouring and spreading emerging orthotospoviruses that are quarantine pests for Australia.
pest free areas, pest free places of production or pest free production sites; or
fruit treatment (such as irradiation)
addition of Appendix C ‘Stakeholder comments’, which addresses a technical issue raised by a stakeholder in this final report.
Introduction
Australia’s biosecurity policy framework
Australia’s biosecurity policies aim to protect Australia against the risks that may arise from exotic pests entering, establishing and spreading in Australia, thereby threatening Australia’s unique flora and fauna, as well as those agricultural industries that are relatively free from serious pests.
Further information about Australia’s biosecurity framework is provided in the Biosecurity Import Risk Analysis Guidelines 2016, located on the department website at agriculture.gov.au/biosecurity-trade/policy/risk-analysis/guidelines.
This risk analysis
Background
The Indian Government Department of Agriculture and Farmers Welfare formally requested market access to Australia for fresh okra fruit for human consumption in a submission received in February 2017. This submission provided information on the pests associated with okra in India, including the plant parts affected. Information was also provided on the standard commercial production practices for okra in India.
Scope
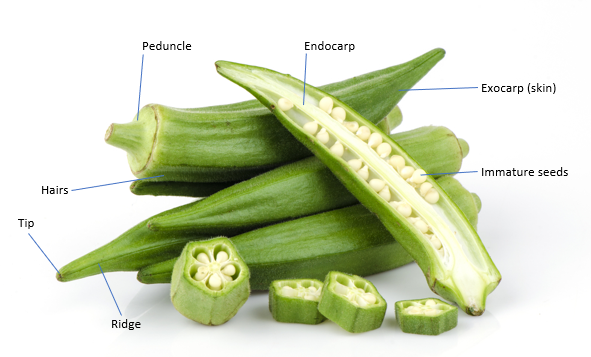
Figure 1.1 Morphology of okra fruit
Existing policy
International policy
The department has reviewed all the pests and pest groups previously identified in existing policies and, where relevant, the information in those assessments has been considered in this risk analysis. The department has also reviewed the latest scientific literature and other information to ensure that the previous assessments are still valid.
The biosecurity risk posed by thrips and the orthotospoviruses they transmit was previously assessed for all countries in the Final group pest risk analysis for thrips and orthotospoviruses on fresh fruit, vegetable, cut-flower and foliage imports (thrips Group PRA) (DAWR 2017).
Domestic arrangements
Contaminating pests
In addition to the pests of okra from India that are assessed in this risk analysis, other organisms may arrive with the imported commodity. These organisms may include pests considered not to be associated with the fruit pathway, pests of other crops, or predators and parasitoids of arthropods. The department considers these organisms to be contaminating pests (‘contaminants’) that could pose sanitary (to human or animal life or health) or phytosanitary (to plant life or health) risks. These risks are identified and addressed using existing operational procedures that require an inspection of all consignments during processing and preparation for export. Consignments will also undergo a verification process on arrival in Australia. The department will investigate whether any pest identified through import verification processes may be of biosecurity concern to Australia and may thus require remedial action.
Consultation
On 21 February 2021, the department notified stakeholders, in Biosecurity Advice 2021-P02, of the commencement of a review of biosecurity import requirements to assess a proposal by India for market access to Australia for okra for human consumption.
Overview of this pest risk analysis
A pest risk analysis (PRA) is 'the process of evaluating biological or other scientific and economic evidence to determine whether an organism is a pest, whether it should be regulated, and the strength of any phytosanitary measures to be taken against it' (FAO 2017a). A pest is ‘any species, strain or biotype of plant, animal, or pathogenic agent injurious to plants or plant products’ (FAO 2022). This definition is also applied in the Biosecurity Act 2015.
The department conducted this PRA in accordance with Australia’s method for pest risk analysis (Appendix A), which is consistent with the International Standards for Phytosanitary Measures (ISPMs), including ISPM 2: Framework for pest risk analysis (FAO 2019a) and ISPM 11: Pest risk analysis for quarantine pests (FAO 2019b), and the WTO Agreement on the Application of Sanitary and Phytosanitary Measures (the SPS Agreement) (WTO 1995).
Initiation—identification of:
the pathway being assessed in the risk analysis
Pest risk management—the process of identifying and proposing/recommending required phytosanitary measures to reduce the biosecurity risk to achieve the ALOP for Australia where the URE is determined as not achieving the ALOP for Australia. Restricted risk is estimated with these phytosanitary measure(s) applied.
A phytosanitary measure is ‘any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non-quarantine pests’ (FAO 2022).
initiation and pest categorisation see: Appendix B
pest risk assessments for pests/pest groups identified in Appendix B as requiring further pest risk assessment see: Chapter 3
Next steps
Commercial production practices for okra in India
Considerations used in estimating unrestricted risk
India provided a technical market access submission to Australia that included information on commercial production practices of okra in India.
Officers from the department visited okra farms, packing houses and treatment (e.g., irradiation) facilities in the Indian states of Maharashtra and Gujarat in December 2022. The objective of the visit was to observe commercial production, pest management and other export practices. The observations during the visit and additional information provided confirmed the production and packing procedures described in this chapter as standard commercial production practices for okra for export from India.
Production areas of okra
Climate in production areas
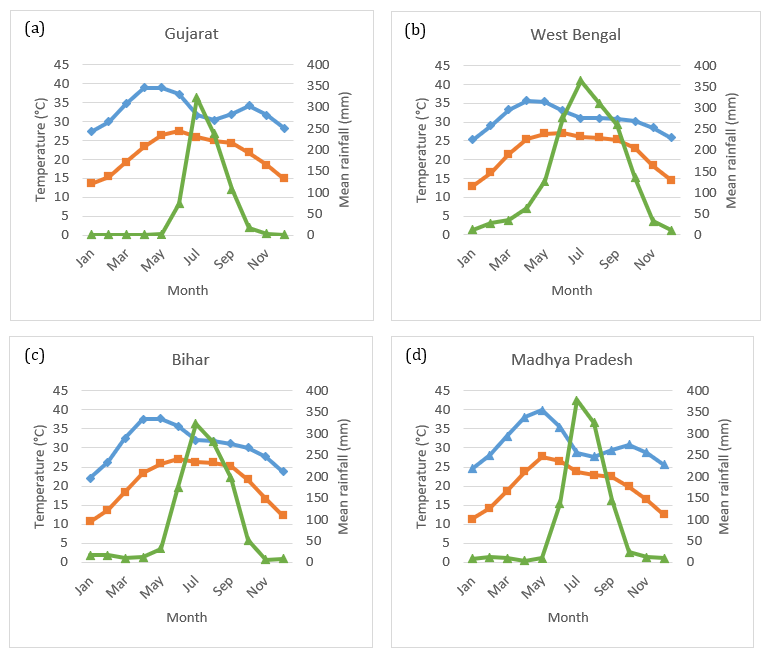
India has a wide range of climatic conditions, including high-rainfall tropical areas in the south-west, temperate conditions in the north to north-east, montane-alpine environments in the far north and arid to semi-arid areas in the central-western regions (Beck et al. 2018).
The 4 seasons experienced in India are:
As a result of the large geographic range of India, different parts of the country experience different ranges of temperature and rainfall even during the same month or season.
Okra is grown in tropical, sub-tropical and warm temperate regions, with year-round production in the states of Gujarat, Odisha and West Bengal (APEDA 2015). Okra is highly susceptible to low temperatures and frost, failing to germinate at temperatures below 20°C (Reddy 2019a). Temperatures above 42°C slow plant and fruit growth (Dhankhar & Mishra 2005).
Figure 2.1 Mean monthly minimum and maximum temperatures and mean monthly rainfall in the main okra production states in India
Pre-harvest
Cultivars
A brief description of the fruit and yield potential of the widely grown okra varieties developed in India is given in Table 2.1.
Table 2.1 Main commercial okra varieties cultivated in India
| Variety | Characteristics |
|---|---|
| Arka Abhay | Fruit of medium length with 5 ridges, dark green and without hairs. Field tolerant to Okra yellow vein mosaic virus (OYVMV). Average yield potential is 18 t/ha. |
| Arka Anamika | Fruit of medium length with 5–6 ridges, dark green and without hairs. Moderately resistant to OYVMV. Average yield potential is 20 t/ha. |
| Kashi Bhairav | Fruit 10–12 cm length with 5 ridges and dark green. Resistant to OYVMV and Okra leaf curl virus (OLCV) under field conditions. Yield potential is 20–22 t/ha. |
| Kashi Kranti | Fruit 8–10 cm in length with 5 ridges and light green. Resistant to OYVMV and OLCV. Yield potential is 12.5–14 t/ha. |
| Kashi Pragati | Fruit 10–12 cm in length with 5 ridges, light green and without hairs. Resistant to OYVMV and OLCV. Yield potential is 15–18 t/ha. |
| Kashi Satdhari | Fruit 13–15 cm in length with 7 ridges and without hairs. Resistant to OYVMV under field conditions. Yield potential is 11–14 t/ha. |
| Parbhani Kranti | Fruit 10–12 cm in length with 5 ridges, dark green, slender and with hairs. Field tolerant to OYVMV. Yield potential is 9–11.5 t/ha. |
| Punjab 7 | Fruit of medium length with 5 ridges, dark green and without hairs. Resistant to OYVMV. Average potential yield is 11.2 t/ha. |
| Punjab Padmini | Fruit 15–20 cm in length with 5 ridges, dark green and without hairs. Yield potential is 10–12 t/ha. |
| Pusa A-4 | Fruit 12–15 cm in length with 5 ridges, dark green and without hairs. Resistant to OYVMV. Average yield potential is 14 t/ha. |
| Pusa Mukhamali | Fruit 15–20 cm in length with 5 ridges, light green and without hairs. Highly susceptible to OYVMV. Yield potential is 8–10 t/ha. |
| Pusa Sawani | Fruit 15–20 cm in length with 5 ridges and dark green. Susceptible to OYVMV. Yield potential is 12–15 t/ha. |
| Varsha Uphar | Fruit of medium length with 5 ridges, dark green and without hairs. Average yield potential is 9.8 t/ha. |
Cultivation practices
Planting season
Farm preparation and planting
Figure 2.2 Okra crop using plastic mulch to preserve water and manage weed growth

Pest management
Okra fields are registered with the respective state agriculture department and crop management is supervised by the respective state agriculture department and the National Plant Protection Organisation (NPPO), the Department of Agriculture and Farmers Welfare (DAFW). Official inspections are undertaken in the place of production at appropriate times during the growing season to check for the presence of pests and diseases in okra crops. Field inspections are primarily carried out by officials from the respective state agriculture department and, if required, by officials from DAFW (Government of India 2021).
Okra farmers implement a wide range of pest control regimes. Chemical control and cultural practices are commonly applied in an integrated program to reduce pest incidence, and bio-control agents such as Beauveria bassiana may also be used (Government of India 2017a; Kedar, Kumerang & Thodsare 2013; Sushil et al. 2020). Government programs are in place that aim to educate farmers in the proper use of control techniques and integrated pest management procedures (Satyagopal et al. 2014). Surveillance of pest and disease hotspots is undertaken periodically by private institutions and by state and federal government officials (Government of India 2017a). Table 2.2 outlines some control methods used for common pests of okra.
Table 2.2 Example of pest management techniques for okra in India
Source: Chittora and Singh (2016); Government of India (2017a); Samnotra et al. (2016); Sushil et al. (2020)
Harvesting and handling procedures

Source: Tuskegee University (2009)
Post-harvest
Source: Infonet (2019)
Packing houses which receive okra intended for export are inspected and certified by the Pack House Inspection Committee constituted by the Agricultural and Processed Food Products Export Development Authority (APEDA). The Pack House Inspection Committee consists of a member of the horticulture division from APEDA head office, a member from the regional APEDA office, a member from the Directorate of Marketing and Inspection, and a member of the state agriculture department (APEDA 2014). To obtain certification, a packing house must meet prescribed standards of quarantine safety, including a separate plant quarantine area for phytosanitary inspection at the point of export (APEDA 2014). The suitability of packing house infrastructure for safe commodity handling and storage, including facilities for pre-cooling and cool storage, are covered by the certification process, and internal quality assurance systems are validated for storage and hygiene practices, and record keeping and traceability (APEDA 2014).
Packing house processes
Sorting and grading
Figure 2.6 Okra being sorted and graded

Packing

Source: Government of India (2021)
Phytosanitary inspection
Transport
Export capability
Production statistics
India is the largest producer of okra in the world, producing 6,075,900 t during the 2017–18 growing season. A summary of okra production for major okra producing Indian states is provided in Table 2.3.
Table 2.3 Okra production in major okra producing states of India (2017-18 growing season)
| State | Yield (tonnes) |
|---|---|
| Gujarat | 921,720 |
| West Bengal | 914,860 |
| Bihar | 787,780 |
| Madhya Pradesh | 638,340 |
| Odisha | 566,880 |
| Chhattisgarh | 323,340 |
| Uttar Pradesh | 307,290 |
| Haryana | 233,960 |
| Andhra Pradesh | 205,910 |
| Telangana | 167,260 |
Export statistics
Export season
| Gujarat | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| West Bengal | ||||||||||||||||||||||||
| Bihar |
| Odisha |
|---|
| Uttar Pradesh |
|---|
| Andhra Pradesh | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Telangana |
Peak (■), lean (■), and year-round (■) growing seasons in major okra producing states. Adapted from: APEDA (2015)
Pest risk assessments for quarantine pests
Summary of outcomes of pest initiation and categorisation
The initiation process (Appendix B) identified 219 pests as being associated with okra in India.
In applying the Group PRAs, 3 thrips, 2 mealy bugs and a scale insect were identified on the import pathway and listed in the pest categorisation (Appendix B). However, if any other quarantine thrips or regulated articles, mealy bugs or scale insects not included in this risk analysis and/or in the respective Group PRAs are detected at pre-export inspection or on arrival in Australia, the appropriate Group policy will also apply to those pests. The application of the Group PRAs to this risk analysis is outlined in Appendix A in section A2.7.
Pests requiring further pest risk assessment
The 10 pests, associated with commercially produced okra for export from India, identified as requiring further pest risk assessment, are listed in Table 3.1. Of these 10 pests:
Table 3.1 Quarantine pests and regulated articles associated with okra from India, and requiring further pest risk assessment
Overview of pest risk assessment
This chapter assesses, for each of the pests or pest groups identified in Table 3.1, the likelihoods of entry, establishment and spread, and the magnitude of the associated potential consequences these species may cause if they were to enter, establish and spread in Australia.
All of the pests or pest groups in Table 3.1 have been assessed previously by the department. Where appropriate, the outcomes of the previous assessments for these pests have been adopted for this risk analysis, unless new information is available that suggests the risk would be different. The acronym ‘EP’ is used to identify species assessed previously and for which import policy already exists. The process relating to the adoption of outcomes from previous assessments is outlined in Appendix A in section A2.6.
A summary of the likelihood, consequence and URE ratings obtained in each pest risk assessment is provided in Table 3.8. An overview of the decision process at the initiation, pest categorisation and pest risk assessment stages of this PRA is presented diagrammatically in Figure 3.1.
Peach fruit fly and melon fly
Bactrocera zonata (EP) and Zeugodacus cucurbitae (EP)
Bactrocera zonata (peach fruit fly) and Zeugodacus cucurbitae (melon fly) belong to the Tephritidae family, a group of fruit flies considered to be among the most damaging pests of horticultural crops. These fruit fly species have not been reported in Australia and therefore are quarantine pests for all of Australia.
Bactrocera zonata has been assessed previously in the existing policies for pomegranates from India (DAWE 2020a) and mangoes from Indonesia, Thailand and Vietnam (DAWR 2015). Zeugodacus cucurbitae has been assessed previously in existing policies (as B. cucurbitae and Z. cucurbitae) in jujubes from China (Department of Agriculture 2020), lychees from Taiwan and Vietnam (DAFF 2013), and longans and lychees from China and Thailand (DAFF 2004). In those policies, the UREs for B. zonata and Z. cucurbitae were assessed as not achieving the ALOP for Australia and specific risk management measures were required.
The current assessment of these fruit flies builds on the previous assessments. However, there may be differences in commercial production practices, climatic conditions, fruit biology, and pest prevalence between the previously assessed commodity/country pathways and okra from India. These differences make it necessary to reassess the likelihood that these fruit flies will be imported into Australia with okra from India.
Likelihood of entry
The likelihood of entry is considered in 2 parts: the likelihood of importation and the likelihood of distribution, which consider pre-border and post-border issues, respectively.
Likelihood of importation
Bactrocera zonata and Z. cucurbitae are present in India, and India produces okra throughout the year (EPPO 2021; Government of India 2017a).
Okra has been reported to be a viable host for B. zonata and Z. cucurbitae, although fruit fly infestation of okra in the field has never been reported in India and is rarely reported in other countries (El-Gendy 2017; Kumagai, Tsuchiya & Katsumata 1996; Syed, Ghani & Murtaza 1970; Wong et al. 1989).
Okra grows best in temperatures of 22°C to 35°C (Government of India 2017a), which are favourable temperatures for the development of fruit flies. Therefore, it is possible that fruit flies could infest okra in India prior to harvest.
There are no reports available on what stage(s) of okra fruit (e.g., immature, mature and/or hardened) is able to be infested by these fruit flies, considering okra is harvested when fruit are immature.
Fruit fly eggs and larvae may remain viable during cold transport and storage.
The development time of fruit flies is inversely dependent on temperature, with development time increasing at lower ambient temperature (Duyck, Sterlin & Quilici 2004; Fletcher 1989; Mkiga & Mwatawala 2015).
Likelihood of distribution
The likelihood that the assessed fruit flies will be distributed within Australia in a viable state as a result of the processing, sale or disposal of okra from India, and subsequently transfer to a susceptible part of a host, is likely to be similar to B. zonata and Z. cucurbitae on previously assessed pathways. The same rating of High for the likelihood of distribution for these fruit flies in previous assessments is adopted for okra from India.
Likelihoods of establishment and spread
Overall likelihood of entry, establishment and spread
The overall likelihood of entry, establishment and spread is determined by combining the individual likelihoods of entry, of establishment and of spread using the matrix of rules in Table A.2.
The overall likelihood that fruit flies will enter Australia as a result of trade in okra from India, be distributed in a viable state to a susceptible part of a host, establish in Australia and subsequently spread within Australia is assessed as Very Low.
Consequences
Unrestricted risk estimate
| Unrestricted risk estimate for B. zonata and Z. cucurbitae | |
|---|---|
| Overall likelihood of entry, establishment and spread | Very Low |
| Consequences | High |
| Unrestricted risk | Low |
Papaya mealybug and Madeira mealybug
Two mealybug species on the okra from India pathway, Paracoccus marginatus (papaya mealybug) and Phenacoccus madeirensis (Madeira mealybug), were identified as quarantine pests for Australia.
The indicative likelihood of entry for all mealybug species is assessed in the mealybugs Group PRA as Moderate (DAWR 2019). Phenacoccus marginatus and P. madeirensis are reported from India and have been associated with okra (Ben-Dov 1994; Kedar, Kumerang & Thodsare 2013; Sakthivel et al. 2012; Shylesha & Joshi 2012). Standard packing house processes and transportation are not expected to eliminate these mealybugs from the pathway. After assessment of relevant pathway-specific factors (sections A2.6 and A2.7) for okra from India, likelihoods of entry of Moderate were verified as appropriate for these mealybug species on this pathway (Table 3.2).
| Risk component | Rating for quarantine mealybugs |
|---|---|
| Likelihood of entry (importation x distribution) | Moderate (High x Moderate) |
| Likelihood of establishment | High |
| Likelihood of spread | High |
| Overall likelihood of entry, establishment and spread | Moderate |
| Consequences | Low |
| Unrestricted risk | Low |
This risk assessment, which is based on the mealybugs Group PRA, applies to all quarantine mealybugs on the okra from India pathway, irrespective of their specific identification in this document. This process is further described in section A2.7.
Mulberry scale
Pseudaulacaspis pentagona (GP, WA)
As assessed in the scale insects Group PRA, the indicative URE for scale insects is Low (Table 3.4), which does not achieve the ALOP for Australia. This indicative URE is considered to be applicable for the quarantine scale insects on the okra from India pathway. Therefore, specific risk management measures are required for the quarantine scale insect pests on this pathway.
This risk assessment, which is based on the scale insects Group PRA, applies to all quarantine scale insects on the okra from India pathway, irrespective of their specific identification in this document. This process is further described in section A2.7.
Eurasian flower thrips, chilli thrips and melon thrips
Scirtothrips dorsalis is present in Australia and is not under official control and, therefore, is not a quarantine pest for Australia.
Frankliniella intonsa, S. dorsalis and T. palmi are identified as regulated articles because they are capable of harbouring and spreading (vectoring) emerging orthotospoviruses that are quarantine pests for Australia, as detailed in the thrips Group PRA (DAWR 2017).
| Pest | In thrips Group PRA | Quarantine pest | Regulated thrips | On okra pathway | Likelihood of entry |
|---|---|---|---|---|---|
| Frankliniella intonsa | Yes | Yes | Yes | Yes | Moderate |
| Thrips palmi | Yes | Yes (SA, WA) | Yes | Yes | Moderate |
| Scirtothrips dorsalis | Yes | No | Yes | Yes | Moderate |
A summary of the risk assessment for quarantine thrips is presented in Table 3.6 for convenience.
Table 3.6 Risk estimates for quarantine thrips
| Risk component | Rating for emerging quarantine orthotospoviruses (a) |
|---|---|
| Likelihood of entry (importation x distribution) | Low (Moderate x Moderate) |
| Likelihood of establishment | Moderate |
| Likelihood of spread | High |
| Overall likelihood of entry, establishment and spread | Low |
| Consequences | Moderate |
| Unrestricted risk | Low |
As assessed in the thrips Group PRA, the URE for emerging quarantine orthotospoviruses transmitted by regulated thrips is Low (Table 3.7), which does not achieve the ALOP for Australia.
This URE is considered to be applicable for the emerging orthotospoviruses known to be vectored by the thrips species present on the okra from India pathway. Therefore, specific risk management measures are required for the regulated thrips to mitigate the risks posed by emerging quarantine orthotospoviruses.
Okra spider mite and okra mite
Tetranychus macfarlanei has been reported from India, Bangladesh, Madagascar, Mauritius and the Canary Islands (Bolland, Gutierrez & Flechtmann 1998; Jeppson, Keifer & Baker 1975; Ullah et al. 2012; Vacante 2016). Tetranychus truncatus is widely distributed in Southeast Asia, including India (Bachhar et al. 2019; Srinivasan et al. 2012) and Indonesia, and extends to Japan and Korea in the east, and to Iran in the west (Bolland, Gutierrez & Flechtmann 1998; Vacante 2016).
Tetranychid mites have 5 distinct life stages: egg, larva, protonymph, deutonymph and adult. At the end of the active larval stage there is a quiescent phase called nymphochrysalis, and at the completion of each nymphal stage, the quiescent phases are deutochrysalis and teliochrysalis (Sakunwarin, Chandrapatya & Baker 2003). After the teleiochrysalis quiescent phase, the deutonymph moults into the adult stage.
Tetranychus macfarlanei and T. truncatus have not been previously assessed by the department. However, a pest group of tetranychid mites has previously been assessed by the department and import policies for tetranychid mites already exist. Tetranychus canadensis, T. mcdanieli, T. pacificus and T. turkestani have been assessed in the final import risk analysis report for stone fruit from California, Idaho, Oregon and Washington (stone fruit from the USA) (Biosecurity Australia 2010).
Tetranychus macfarlanei and T. truncatus have similar biological characteristics to 2 of those spider mite species - T. pacificus and T. turkestani - including:
The assessment of spider mites on stone fruit from the USA (Biosecurity Australia 2010) rated the likelihood of distribution as Moderate. Okra fruit are expected to be distributed in Australia as a result of the processing, sale or disposal of the imported produce in a similar way to stone fruit from the USA. Fruit that are unmarketable are likely to be disposed of as municipal waste, from where it is unlikely that spider mites will be distributed into the environment. From domestic situations, fruit waste disposed of as litter may be deposited into urban or peri-urban situations, as well as areas of natural vegetation. Spider mites on both pathways have a polyphagous habit. They can infest a wide range of agricultural and horticultural crops and hosts that can be found in domestic gardens, as well as in urban environments as amenity plants or weeds. Therefore, the time of year when importation occurs will not affect the likelihood of distribution for these spider mites. On the basis outlined, the likelihood of distribution of Moderate previously assessed for spider mites on the stone fruit from the USA pathway has been adopted for spider mites on the okra fruit from India pathway.
The likelihoods of establishment and spread of spider mites on okra from India will be comparable with spider mites on stone fruit from the USA because these likelihoods relate specifically to events that occur in Australia and are independent of the import pathway. The consequences of entry, establishment and spread of spider mites in Australia are also independent of the import pathway. The existing ratings for the likelihoods of establishment and spread, and the rating for the overall consequences for spider mites on the stone fruit from the USA pathway have been adopted for spider mites on the okra from India pathway.
Likelihood of entry
The likelihood that the assessed spider mites will arrive in Australia in a viable state with the importation of okra from India is assessed as High.
The likelihood of importation is assessed as High because T. macfarlanei and T. truncatus are present in India and the okra plant is known to be a viable host for completion of development. Okra is a perishable fruit that requires careful handling during post-harvest processing to avoid damage to the fruit surface. Therefore, spider mite adults, juveniles or eggs residing on the fruit surface may not be dislodged during postharvest handling. Sorting and grading procedures in packing houses may not detect and remove these development stages as they are small and difficult to observe without a magnification device such as a hand lens. Some adults, nymphs or eggs may survive the low temperatures during storage and transportation of okra. Various spider mite species have been intercepted numerous times on imported fresh produce on arrival in Australia.
Okra grows best at temperatures of 22°C to 35°C, which covers the optimal temperature ranges for development of T. macfarlanei (Borkar, Kolhe & Undirwade 2020; Latha et al. 2019; Ullah et al. 2012) and T. truncatus (Sakunwarin, Chandrapatya & Baker 2003; Win et al. 2018). All immature stages and adults would likely be present in okra fields during pod development and harvest.
Spider mites are primarily pests of leaves but can also be found on fruit. Spider mite adults, juveniles or eggs present on fruit are unlikely to be completely removed during harvesting and post-harvest processes.
Okra fruit provide some points, such as the remnant of the peduncle, and base of ridges and spines/hairs on the surface (often present on heirloom varieties), where spider mites may reside.
Storage and transport conditions are unlikely to kill all life stages of spider mites.
The larval period is relatively short, up to 2 days (Borkar, Kolhe & Undirwade 2020; Pang et al. 2004; Sakunwarin, Chandrapatya & Baker 2003), and larvae enter the nymphochrysalis quiescent stage in a shorter time as ambient temperatures increase (Ullah et al. 2012; Win et al. 2018). Following their emergence from eggs on the leaves, larvae need to migrate to fruit and are unlikely to survive an extended period of cool conditions during postharvest storage.
Eggs are not reported to be laid on okra fruit. However, at high population densities, adult females may disperse to the fruit and incidental egg laying may occur. At low temperatures (7°C to 10°C), the viability of eggs is low and the mortality rate of emerging larvae is high (Sakunwarin, Chandrapatya & Baker 2003; Ullah et al. 2012).
The likelihood that the assessed spider mites will be distributed within Australia in a viable state as a result of the processing, sale or disposal of okra from India and subsequently transfer to a susceptible part of a host, is likely to be similar to the spider mite species previously assessed on the stone fruit from the USA (Biosecurity Australia 2010). The same rating of Moderate for the likelihood of distribution for spider mite species in the previous assessment is adopted for the assessed spider mites for okra from India.
Overall likelihood of entry
Likelihoods of establishment and spread
Overall likelihood of entry, establishment and spread
The overall likelihood that spider mites will enter Australia as a result of trade in okra fruit from India, be distributed in a viable state to a susceptible part of a host, establish in Australia and subsequently spread within Australia is assessed as Moderate.
Consequences
The potential consequences of the entry, establishment and spread of T. macfarlanei and T. truncatus in Australia are similar to those in the previous assessments of spider mite species for stone fruit from the USA (Biosecurity Australia 2010). The overall consequences in the previous assessments were assessed as Low. The overall consequences for spider mites on the okra from India pathway are also assessed as Low.
Unrestricted risk estimate
Pest risk assessment conclusions
The UREs for the 9 quarantine pests were assessed as not achieving the ALOP for Australia, and thus specific risk management measures are required for these quarantine pests on this pathway. These pests are:
peach fruit fly (Bactrocera zonata)
Eurasian flower thrips (Frankliniella intonsa)
melon thrips (Thrips palmi)
Table 3.8 Pest risk assessment conclusions for pests, and pest groups, associated with the pathway of okra from India
| Likelihood of | Consequences | URE | ||||||
|---|---|---|---|---|---|---|---|---|
| Pest name | Entry | Establishment | Spread | EES | ||||
| Importation | Distribution | Overall | ||||||
| Fruit flies (Diptera: Tephritidae) | ||||||||
| Bactrocera zonata (EP) | Very Low | High | Very Low | High | High | Very Low | High | Low |
| Zeugodacus cucurbitae (EP) | Very Low | High | Very Low | High | High | Very Low | High | Low |
| Mealybugs (Hemiptera: Pseudococcidae) | ||||||||
| Paracoccus marginatus (GP) | High | Moderate | Moderate | High | High | Moderate | Low | Low |
| Phenacoccus madeirensis (GP) | High | Moderate | Moderate | High | High | Moderate | Low | Low |
| Scale insect (Hemiptera: Diaspididae) | ||||||||
| Pseudaulacaspis pentagona (GP, WA) | High | Moderate | Moderate | High | High | Moderate | Low | Low |
| Thrips (Thysanoptera: Thripidae) | ||||||||
| Frankliniella intonsa (GP) a | High | Moderate | Moderate | High | High | Moderate | Low | Low |
| Scirtothrips dorsalis (GP, RA) | High | Moderate | Moderate | N/A | N/A | N/A | N/A | N/A |
| Thrips palmi (GP, SA, WA) a | High | Moderate | Moderate | High | High | Moderate | Low | Low |
| Spider mites (Trombidiformes: Tetranychidae) | ||||||||
| Tetranychus macfarlanei | High | Moderate | Moderate | High | High | Moderate | Low | Low |
| Tetranychus truncatus | High | Moderate | Moderate | High | High | Moderate | Low | Low |
| Orthotospoviruses [Bunyavirales: Tospoviridae] vectored by Frankliniella intonsa (a), Scirtothrips dorsalis (RA) and Thrips palmi (a) | ||||||||
| Listed in the thrips group PRA (GP) | Moderate | Moderate | Low | Moderate | High | Low | Moderate | Low |
EP: Species has been assessed previously and import policy already exists. GP: Species has been assessed previously in a Group PRA and the Group PRA has been applied. RA: Regulated article. WA: Regional quarantine pest for Western Australia. SA: Regional quarantine pest for South Australia. EES: Overall likelihood of entry, establishment and spread. URE: Unrestricted risk estimate. a: Quarantine thrips species that is also identified as a regulated article for Australia as it can vector emerging quarantine orthotospoviruses, and this table presents the risk estimates for these viruses from the thrips Group PRA (DAWR 2017a). N/A: not applicable, as S. dorsalis is present in Australia and is not a quarantine pest.
Pest risk management
Pest risk management measures and phytosanitary procedures
Historical trade and pest interception data of other similar pathways, as described in section 4.1.1, have been considered in determining the appropriate risk management measures for the importation of okra from India.
Analysis of pest interception data
Australia currently allows imports of fresh okra fruit from Fiji. However, there have been no imports of okra from Fiji since 2018. Between 2013 and 2017, Fiji exported a total of 3.6 t of okra to Australia. Interception data of okra from Fiji showed 2 detections of larvae of noctuid moths, which were appropriately actioned.
Risk management measures for quarantine pests and regulated articles associated with okra from India
a: PFA is pest free area, PFPP is pest free place of production and PFPS is pest free production site. b: Remedial action may include treatment of the consignment to ensure that the pest is no longer viable, or withdrawal of the consignment from export to Australia. c: Quarantine thrips species that is also identified as a regulated article for Australia as it vectors emerging quarantine orthotospoviruses, assessed in the thrips Group PRA (DAWR 2017) as posing an unrestricted risk that does not achieve the ALOP for Australia. EP: Species has been assessed previously and import policy already exists. RA: Regulated article. GP: Species has been assessed previously in a Group PRA and the Group PRA has been applied. SA: Regional quarantine pest for South Australia. WA: Regional quarantine pest for Western Australia.
for mealybugs, scale insects, thrips and spider mites
pre-export visual inspection and, if detected, remedial action.
Measures for fruit flies
Recommended measure 1: Pest free area, pest free place of production or pest free production site
Recommended measure 2: Fruit treatment such as irradiation
Fruit treatment known to be effective for all life stages of fruit flies such as irradiation applied pre-export may be used as a phytosanitary measure for B. zonata and Z. cucurbitae. The requirements for using irradiation as a phytosanitary measure are set out in ISPM 18: Guidelines for the use of irradiation as a phytosanitary measure (FAO 2019c). Irradiation is recognised as an effective method for pest risk management when performed in approved facilities and at specific dose rates recognised as effective for target pest groups. Food Standards Australia New Zealand permits irradiation dose rates up to a maximum of 1000 gray for quarantine purposes for fresh fruits and vegetables including okra (FSANZ 2017).
The department recommends a treatment schedule of 150 gray minimum absorbed dose, consistent with ISPM 28 Annex 7: Irradiation treatment for fruit flies of the family Tephritidae (generic) (FAO 2017b) for B. zonata and Z. cucurbitae.
Measures for mealybugs, scale insects, thrips and spider mites
Recommended measure: Pre-export visual inspection and, if found, remedial action
Consideration of alternative measures
Operational system for the assurance, maintenance and verification of phytosanitary status
A system of operational procedures is necessary to ensure recommended specific risk management measures (section 4.1) are effectively applied, the phytosanitary status of okra from India is maintained, and these can be verified.
A system of traceability to source farms
The objectives of this recommended procedure are to ensure that:
Where a pest risk management measure involving pest monitoring and controls during production and at harvest (such as PFA, PFPP, PFPS or systems approach) is used, export farms must be registered with DAFW before commencement of each harvest season. Records of registered farms and DAFW audits must be kept by DAFW and must be made available to the department upon request.
Registration of packing houses and treatment providers, and auditing of procedures
The objectives of this recommended procedure are to ensure that:
The approval of treatment providers by DAFW must include verification that suitable systems are in place to ensure compliance with treatment requirements. This may include:
documented procedures to ensure okra are appropriately treated and safeguarded post treatment
The department provides final approval of facilities, following review of regulatory oversight provided by DAFW and the capability demonstrated by the facility. Site visits may be required for the department to have assurance that treatment can be applied accurately and consistently.
The department requires final approval for irradiation facilities.
Packaging, labelling and containers
secure packaging is used for export of okra from India to Australia to prevent re-infestation during storage and transport and prevent escape of pests during clearance procedures on arrival in Australia. Packaging must meet Australia’s secure packing options published on BICON
consignments are made insect-proof and secure by using at least one of the following secure consignment options:
produce transported in fully enclosed containers: cartons (packages) with holes as loose boxes or on pallets may be transported in fully enclosed containers. Enclosed containers include 6-sided containers with solid sides, or ULDs with tarpaulin sides that have no holes or gaps. The container must be transported to the inspection point intact.
packaged okra from India must be labelled with sufficient identification for the purposes of traceability. This may include:
Export packing houses and treatment providers (where applicable) must ensure packaging and labelling are suitable to maintain phytosanitary status of the export consignments.
Specific conditions for storage and movement
The objective of this recommended procedure is to ensure that the quarantine integrity of the okra is maintained during storage and movement.
Freedom from trash
Pre-export phytosanitary inspection and certification by the Indian Government Department of Agriculture and Farmers Welfare
All consignments must be inspected prior to export in accordance with official procedures for all visually-detectable quarantine pests and regulated articles (including trash). Sampling and inspection methods should be consistent with ISPM 23 (FAO 2019d) and ISPM 31 (FAO 2016b) and provide a 95% level of confidence that infestation greater than 0.5% will be detected. For a consignment equal to or greater than 1 000 units (one unit being a single okra fruit), this is equivalent to a 600-unit sample randomly selected across the consignment. Any netting or artificial wrapping material must be removed during the inspection. The inspection technique must be capable of detecting all life stages of quarantine pests.
A phytosanitary certificate must be issued for each consignment upon completion of pre-export inspection and treatment to verify that the required risk management measures have been undertaken prior to export and that the consignment meets Australia’s import requirements.
Some treatments (such as irradiation) may also require treatment certificates that accompany the phytosanitary certificate. BICON will describe where treatment certificates are required.
Phytosanitary inspection by the Department of Agriculture, Fisheries and Forestry
The objectives of this recommended procedure are to ensure that:
assess documentation to verify that the consignment is as described on the phytosanitary certificate, that required phytosanitary actions have been undertaken, and that product security has been maintained
verify that the biosecurity status of consignments of okra from India meet Australia’s import requirements. When inspecting consignments, the department will use random samples of 600 units, or equivalent per phytosanitary certificate and an inspection method suitable for the commodity.
Remedial action(s) for non-compliance
Other actions, including partial or complete suspension of the import pathway, may be taken depending on the identity and/or importance of the pest intercepted, for example, fruit flies of economic importance or pests for which PFAs, PFPPs or PFPSs are established.
In the event that consignments of okra from India are repeatedly non-compliant, the department may require enhanced risk management measures, including mandatory phytosanitary treatment. The department reserves the right to suspend imports (either all imports, or imports from specific pathways) and to conduct an audit of the risk management systems. Imports will be allowed to recommence only when the department is satisfied that appropriate corrective action has been undertaken.
Uncategorised pests
Review of processes
Verification of protocol
Review of policy
DAFW must inform the department immediately on the detection of any new pests of okra in India that might be of potential biosecurity concern to Australia.
Meeting Australia’s food laws
In addition to meeting Australia’s biosecurity laws, imported food for human consumption must comply with the requirements of the Imported Food Control Act 1992, as well as Australian state and territory food laws. Among other things, these laws require all food, including imported food, to meet the standards set out in the Australia New Zealand Food Standards Code (the Code).
Certain imported food, including some minimally processed horticulture products, must be covered by a food safety management certificate to be imported into Australia. The certificate provides evidence that a food has been produced through a food safety management system. This system must have appropriate controls in place to manage food safety hazards. More information about the foods that require a food safety management certificate and how to comply is available at agriculture.gov.au/biosecurity-trade/import/goods/food/lodge/safety-management-certificates.
Conclusion
This final risk analysis report was conducted to assess the proposal by India for market access to Australia for fresh okra fruit for human consumption.
All fresh fruit, including okra fruit from India, have been determined by the Director of Biosecurity to be conditionally non-prohibited goods under s174 of the Biosecurity Act 2015. Conditionally non-prohibited goods cannot be brought or imported into Australia unless they meet specific import conditions.
This report, upon its finalisation, provides the basis for import conditions for fresh okra fruit from India for human consumption. The import conditions will be communicated on BICON. The publication of import conditions on BICON is subject to India being able to demonstrate that processes and procedures are in place to implement the required risk management measures.
Appendix A: Method for pest risk analysis
Restricted risk is estimated with phytosanitary measure(s) applied. A phytosanitary measure is ‘any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non-quarantine pests’ (FAO 2022).
A PRA is conducted in 3 consecutive stages: initiation (A1), pest risk assessment (A2) and pest risk management (A3).
Stage 1: Initiation
the identification of a pathway that presents a potential pest hazard. For example, international trade is requested for a commodity not previously imported into the country or a commodity from a new area or new country of origin
the identification of a pest that may require phytosanitary measures. For example, a new pest risk is identified by scientific research, a pest is repeatedly intercepted, a request is made to import an organism, or an organism is identified as a vector of other pests
potential association of each pest with the pathway being assessed.
The identity of the pests is presented at species level by the species’ scientific name in most instances, but a lower taxonomic level may be used where appropriate. Synonyms are provided where the current scientific name differs from that provided by the exporting country’s National Plant Protection Organisation (NPPO) or where the cited literature used a different scientific name.
Stage 2: Pest risk assessment
pest categorisation (A2.1) and
further pest risk assessment, which includes evaluation of the likelihood of the introduction (entry and establishment) and spread of a pest (A2.2) and evaluation of the magnitude of the associated potential consequences (A2.3).
Pest categorisation
potential for entry, establishment and spread in the PRA area
potential for economic consequences in the PRA area.
Assessment of the likelihood of entry, establishment and spread
Likelihood of entry
Likelihood of importation—the likelihood that a pest will arrive in Australia in a viable state when a given commodity is imported
Likelihood of distribution— the likelihood that the pest will be distributed in a viable state, as a result of the processing, sale or disposal of the commodity, in the PRA area and subsequently transfer to a susceptible part of a host.
mode of trade (for example, bulk, packed)
volume and frequency of movement along each pathway
vulnerability of the life-stages of the pest during transport or storage
prevalence of the pest likely to be associated with a consignment
dispersal mechanisms of the pest, including vectors, to allow movement from the pathway to a suitable host
whether the imported commodity is to be sent to a few or many destination points in the PRA area
Likelihood of establishment
Establishment is defined as the ‘perpetuation for the foreseeable future, of a pest within an area after entry’ (FAO 2022). In order to estimate the likelihood of establishment of a pest, reliable biological information (for example, lifecycle, host range, epidemiology, survival) is obtained from the areas where the pest currently occurs. The situation in the PRA area can then be compared with that in the areas where it currently occurs and expert judgement used to assess the likelihood of establishment.
Factors to be considered in the likelihood of establishment in the PRA area may include:
whether a vector, if needed for dispersal of the pest, is already present in the PRA area or likely to be introduced
suitability of environment in the PRA area
potential for adaptation of the pest
minimum population needed for establishment.
Likelihood of spread
potential for movement with commodities, conveyances or by vectors
intended use of the commodity
Assigning likelihoods for entry, establishment and spread
| Likelihood | Descriptive definition | Indicative range |
|---|---|---|
| High | The event would be very likely to occur | 0.7 < to ≤ 1 |
| Moderate | The event would occur with an even likelihood | 0.3 < to ≤ 0.7 |
| Low | The event would be unlikely to occur | 0.05 < to ≤ 0.3 |
| Very Low | The event would be very unlikely to occur | 0.001 < to ≤ 0.05 |
| Extremely Low | The event would be extremely unlikely to occur | 0.000001 < to ≤ 0.001 |
| Negligible | The event would almost certainly not occur | 0 < to ≤ 0.000001 |
Combining likelihoods
The likelihood of entry is determined by combining the likelihood that the pest will be imported into the PRA area and the likelihood that the pest will be distributed within the PRA area, using a matrix of rules (Table A.2). This matrix is then used to combine the likelihood of entry and the likelihood of establishment, and the likelihood of entry and establishment is then combined with the likelihood of spread to determine the overall likelihood of entry, establishment and spread.
For example, if a descriptor of Low is assigned for the likelihood of importation, Moderate for the likelihood of distribution, High for the likelihood of establishment and Very Low for the likelihood of spread, then the likelihood of importation of Low and the likelihood of distribution of Moderate are combined to give a likelihood of Low for entry. The likelihood for entry is then combined with the likelihood assigned for establishment of High to give a likelihood for entry and establishment of Low. The likelihood for entry and establishment is then combined with the likelihood assigned for spread of Very Low to give the overall likelihood for entry, establishment and spread of Very Low. This can be summarised as:
Time and volume of trade
One factor affecting the likelihood of entry is the volume and duration of trade. If all other conditions remain the same, the overall likelihood of entry will increase as time passes and the overall volume of trade increases.
The department normally considers the likelihood of entry on the basis of the estimated volume of one year’s trade. This is a convenient value for the analysis that is relatively easy to estimate and allows for expert consideration of seasonal variations in pest presence, incidence and behaviour to be taken into account. The consideration of the likelihood of entry, establishment and spread and subsequent consequences takes into account events that might happen over a number of years even though only one year’s volume of trade is being considered. This difference reflects biological and ecological facts, for example where a pest or disease may establish in the year of import but spread may take many years.
Assessment of potential consequences
the life or health of plants and plant products
This may include pest impacts on the life or health of the plants and production effects (yield or quality) either at harvest or during storage.
This may include pest impacts on new or modified eradication, control, surveillance or monitoring and compensation strategies or programs.
domestic trade
This may include pest impacts on the community and environment, including reduced tourism, reduced rural and regional economic viability, loss of social amenity, and any ‘side effects’ of control measures.
For each of these direct and indirect criteria, the level of impact is estimated over 4 geographic levels, defined as:
For each criterion, the magnitude of impact at each of these geographic levels is described using 4 categories, defined as:
Unlikely to be discernible–pest impact is not usually distinguishable from normal day-to-day variation in the criterion
The following are considered during this process:
At each geographic level below 'National', an impact more serious than ‘Minor significance’ is considered at least 'Minor significance' at the level above. For example, a ‘Significant’ impact at the state or territory level is considered equivalent to at least a ‘Minor significance’ impact at the national level.
For each criterion:
the level of impact is estimated over 4 geographic levels: local, district, regional and national
Table A.3 Decision rules for determining the overall consequence rating for each pest
| Rule | The impact scores for consequences of direct and indirect criteria | Overall consequence rating |
|---|---|---|
| 1 | Any criterion has an impact of ‘G’; or more than one criterion has an impact of ‘F’; or a single criterion has an impact of ‘F’ and each remaining criterion an ‘E’. |
Extreme |
| 2 | A single criterion has an impact of ‘F’; or all criteria have an impact of ‘E’. |
High |
| 3 | One or more criteria have an impact of ‘E’; or all criteria have an impact of ‘D’. |
Moderate |
| 4 | One or more criteria have an impact of ‘D’; or all criteria have an impact of ‘C’. |
Low |
| 5 | One or more criteria have an impact of ‘C’; or all criteria have an impact of ‘B’. |
Very Low |
| 6 | One or more but not all criteria have an impact of ‘B’, and all remaining criteria have an impact of ‘A’; or all criteria have an impact of ‘A’. |
Negligible |
Estimation of the unrestricted risk
Once the assessment of the likelihood of entry, establishment and spread and for potential consequences are completed, the unrestricted risk can be determined for each pest or each group of pests. This is determined by using a risk estimation matrix (Table A.4) to combine the estimates of the likelihood of entry, establishment and spread and the overall consequences of pest establishment and spread.
The appropriate level of protection (ALOP) for Australia
Adoption of outcomes from previous assessments
Outcomes of previous risk assessments have been adopted in this assessment for pests for which the risk profile is assessed as comparable to previously assessed situations.
The prospective adoption of previous risk assessment ratings for the likelihood of importation and the likelihood of distribution is considered on a case-by-case basis by comparing factors relevant to the pathway being assessed with those assessed previously. For assessment of the likelihood of importation, factors considered/compared include the commodity type, the prevalence of the pest and commercial production practices in the exporting country/region. For assessment of the likelihood of distribution of a pest the factors considered/compared include the commodity type, the ways the imported produce will be distributed within Australia as a result of the processing, sale or disposal of the imported produce, and the time of year when importation occurs and the availability and susceptibility of hosts at that time. After comparing these factors and reviewing the latest literature, previously determined ratings may be adopted if the department considers the likelihoods for the pathway being assessed to be comparable to those assigned in the previous assessment(s), and there is no new information to suggest that the ratings assigned in the previous assessment(s) have changed.
Application of Group PRAs to this risk analysis
the Final group pest risk analysis for soft and hard scale insects on fresh fruit, vegetable, cut-flower and foliage imports (scales Group PRA) (DAWE 2021).
The Group PRA approach is consistent with relevant international standards and requirements–including ISPM 2: Framework for Pest Risk Analysis (FAO 2019a), ISPM 11: Pest Risk Analysis for Quarantine Pests (FAO 2019b) and the SPS Agreement (WTO 1995). ISPM 2 states that ‘Specific organisms may … be analysed individually, or in groups where individual species share common biological characteristics.’
Stage 3: Pest risk management
Pest risk management describes the process of identifying and implementing phytosanitary measures to manage risks to achieve the ALOP for Australia, while ensuring that any negative effects on trade are minimised.
The conclusions from pest risk assessment are used to decide whether risk management is required and if so, the appropriate measures to be used. Where the unrestricted risk estimate does not achieve the ALOP for Australia, risk management measures are required to reduce this risk to a very low level. The guiding principle for risk management is to manage risk to achieve the ALOP for Australia. The effectiveness of any proposed/recommended phytosanitary measures (or combination of measures) is evaluated, using the same approach as used to evaluate the unrestricted risk. This ensures the restricted risk for the relevant pest or pests achieves the ALOP for Australia.
options ensuring that the area, place or site of production or crop is free from the pest—for example, pest-free area, pest-free place of production or pest-free production site
options for other types of pathways—for example, consider natural spread, measures for human travellers and their baggage, cleaning or disinfestations of contaminated machinery
Appendix B: Initiation and categorisation for pests of okra from India
The Final group pest risk analysis for thrips and orthotospoviruses on fresh fruit, vegetable, cut-flower and foliage imports (DAWR 2017), the Final group pest risk analysis for mealybugs and the viruses they transmit on fresh fruit, vegetable, cut-flower and foliage imports (DAWR 2019) and the Final group pest risk analysis for soft and hard scale insects on fresh fruit, vegetable, cut-flower and foliage imports (DAWE 2021) have been applied in this risk analysis. Application of Group policy involves identification of up to 3 species of each relevant group associated with the commodity pathway. However, if any other quarantine pests or regulated articles not included in this risk analysis and/or in the relevant Group policies are detected at pre-export or on-arrival in Australia, the relevant Group policy will also apply.
The department is aware of the recent changes in fungal nomenclature which ended the separate naming of different states of fungi with a pleomorphic life cycle. However, as the nomenclature for these fungi is in a phase of transition and many priorities of names are still to be resolved, this report uses the generally accepted names and provides alternatively used names as synonyms, where required. The department is also aware of the changes in nomenclature of arthropod species based on the latest morphological and molecular reviews. As official lists of accepted fungus and arthropod names become available, these accepted names will be adopted.
| Potential to enter on pathway | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pest | Present in India | Present within Australia | Potential for importation | Potential for distribution | Potential for establishment and spread | Potential for economic consequences | Pest risk assessment required | |||||||||||
| ARTHROPODS | ||||||||||||||||||
| Coleoptera | ||||||||||||||||||
Indian rose beetle |
Yes (CABI 2022; Emmanuel, Sujatha & Gautam 2010) | No records found | No. Adult Adoretus versutus are leaf defoliators, while soil-dwelling larvae feed on the roots of host plants, humus and detritus (CABI 2022; Waterhouse 1997). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Alcidodes affaber (Aurivillius) |
Yes (TNAU-NAIP 2020) | No records found | No. Larvae of Alcidodes affaber feed inside the shoot of the okra plant (Manjunatha et al. 2017). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Pumpkin beetle |
Yes (CABI-EPPO 1997; PaDIL 2020) | No records found | No. Adult Aulacophora indica feeding causes large holes in the leaves and may defoliate host plants. The larvae feed exclusively on the roots of host plants (Plantwise 2023; Wang et al. 2020). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Aulacophora foveicollis (Lucas, 1849) |
Yes (Luna et al. 2008; Rashid et al. 2014) | No records found | No. Adults are leaf and flower feeders and larvae feed on the roots of the host plant (Luna et al. 2008; Plantwise 2023; Rashid et al. 2014). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Root weevil; Sweet potato weevil |
Yes (CABI 2022; Korada et al. 2010) | Yes, Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld, NSW, Tas., SA, NT (APPD 2022). | No. Cylas formicarius adults are reported to feed on okra leaves and larvae feed on roots and tubers (CABI 2022; Korada et al. 2010; Tara, Sharma & Kour 2010). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Epilachna ocellata (Redtenbacher, 1844) |
Yes (CABI 2022; Government of India 2007; Lal 1990) | No records found | No. Epilachna ocellata is polyphagous, with adults and larvae preferably feeding externally on leaves (Lal 1990). Also, okra is reported as a less preferred host whereas tomato and eggplant are reported as preferred hosts (Lal 1990). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Chrysomelidae] White-spotted leaf beetle |
Yes (Nair et al. 2017) | No records found | No. The beetle usually feeds on leaves and flowers (Nair et al. 2017). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Yes |
No records found | No. Although okra is reported to be a host plant for M. phalerata, adult beetles only feed on the reproductive parts of the plants such as flowers, preventing the development of pods (Durairaj & Ganapathy 2003; Rolania, Yadav & Saini 2016; Sharma & Singh 2018). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
| Yes (Boopathi et al. 2011; Rolania, Yadav & Saini 2016) | No records found | No. Although okra is reported to be a host for M. pustulata (Brice et al. 2017), this beetle lays eggs in the soil and upon hatching larvae feed on soil-dwelling insects. Adults are destructive external feeders on the reproductive parts of plants, reducing fruit setting (Kedar, Kumerang & Thodsare 2013; Nair et al. 2017). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Faust, 1891 [Curculionidae] |
Yes (CABI 2022; Dhamdhere, Bahadur & Misra 1985) | No records found | No. Myllocerus undecimpustulatus adults feed on leaves of host plants and larvae feed on roots (Neal 2017). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (TNAU-NAIP 2020) | No records found | No. Oxycetonia versicolor is reported as a minor pest of okra in India (Daravath, Kasbe & Musapuri 2020; Taggar et al. 2012; TNAU-NAIP 2020). Adults and larvae only feed on buds and flowers of host plants (Taggar et al. 2012). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Pempherulus affinis (Faust, 1898) [Curculionidae] |
Yes (TNAU-NAIP 2020) | No records found | No. Pempherulus affinis is reported as a pest of okra in India. The larvae feed on roots and shoots of okra (TNAU-NAIP 2020). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Okra flea beetle |
Yes (Kelkar et al. 2018) | No records found | No. Podagrica bowringi adult beetles feed on leaves, flowers and flower buds, and larvae feed on roots of okra (Kelkar et al. 2018). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Podagrica fuscicornis (Linnaeus, 1767) |
Yes (Singhal et al. 2018) | No records found | No. Podagrica fuscicornis is reported to be a leaf feeder of okra (Singhal et al. 2018). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Bruchidae] |
Yes (Borowiec 1991) | No records found | No. Spermophagus spp. are reported to lay eggs externally on pods of some other host plants (Delobel & Klaus-Werner 2011; Southgate 1979; Tóth, Vráblová & Cagáň 2001). |
Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Borowiec 1985) | No records found | No. Spermophagus spp. are reported to lay eggs externally on pods of host plants (Delobel & Klaus-Werner 2011; Southgate 1979; Tóth, Vráblová & Cagáň 2001). While Borowiec (1991) lists okra as a host plant for S. kuskai, there is no evidence that this pest lays eggs on pods of okra. Additionally, there is no evidence available for the association between this species and okra fruit in India. | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Trachys herilla Obenberger, 1916 [Buprestidae] |
Yes (TNAU-NAIP 2020) | No records found | No. Trachys herilla is a leaf miner, primarily associated with the leaves of okra. Larvae feed within the leaf mesophyll tissue forming a mine and adults feed on the margins of young okra leaves (Fernando & Bandaranayake 1991; TNAU-NAIP 2020). The eggs of T. herilla are deposited on the leaf surface (Fernando & Bandaranayake 1991). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Dried fruit beetle; Pineapple sap beetle |
Yes (CABI 2022; Dasgupta & Pal 2019; MAF 1999) | Yes. NSW, Qld, NT, Vic., Tas., SA, WA (APPD 2022; Government of Western Australia 2022; James et al. 1993) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Diptera | ||||||||||||||||||
Atherigona orientalis Schiner, 1868 |
Yes (Gupta, Srivastava & Pandey 1991) | Yes. NSW, NT, Qld, WA (APPD 2022; CABI 2022; Government of Western Australia 2022; Pont 1986) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Tephritidae] Oriental fruit fly |
Yes (Balikai, Kotikal & Prasanna 2009) | No. Eradicated from mainland Australia (Hancock et al. 2000) | No. Okra has been reported to be a viable host in a no-choice host assay laboratory study where the emergence rate in whole fruit was very low (Kumagai, Tsuchiya & Katsumata 1996). There are no reports available of B. dorsalis infesting okra in the field. | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Agarwal & Kumar 1999) | No records found | Yes. Okra is reported to be a host of B. zonata (El-Gendy 2017). Bactrocera zonata has been reared on okra in field situations (Syed, Ghani & Murtaza 1970). | Yes. Okra fruit will be distributed across Australia for sale and could potentially carry fruit fly eggs and larvae. Immature stages that could be potentially present in imported okra could pupate and develop into adults and disperse to new hosts available in Australia. | Yes. Bactrocera zonata has suitable hosts and environments available in Australia. This species has established in areas with a wide range of climatic conditions (Alzubaidy 2000). Bactrocera zonata has spread across pan-tropical areas, with a minimum developmental temperature of 13°C (Alzubaidy 2000; Duyck, Sterlin & Quilici 2004). Bactrocera zonata is reported to disperse long distances (Qureshi et al. 1974). | Yes. Bactrocera zonata is highly polyphagous, feeding on over 50 host plants, some of which are commercial crops of economic importance in Australia (Alzubaidy 2000; EPPO 2015). In heavy infestations, total crop losses have been reported (Alzubaidy 2000; Mahmoudi et al. 2017). | Yes (EP) | ||||||||||||
Dacus ciliatus Loew, 1862 |
Yes (Kapoor 2002) | No records found | No. Dacus ciliatus is a pest of cucurbit crops. In a taxonomic study, Munro (1984) listed okra as a host plant but provided no supporting evidence for the host association. In a review, White and Elson-Harris (1994), citing Munro (1984), noted okra as an unusual host. There is no report available of D. ciliatus infesting okra in the field. | Assessment not required. | Assessment not required | Assessment not required | No | |||||||||||
[Anthomyiidae] Cabbage root fly |
Yes (Sharma & Rao 2012) | No records found | No. Delia radicum is primarily a pest of Brassica species. There are reports of this pest feeding on okra seedlings and mature fruit (Ahmed 2012; Sharma & Rao 2012). Delia radicum is not reported to be a pest of concern on okra in India, and highly unlikely to be present in commercially grown export quality okra, as the fruit for consumption is harvested several weeks before reaching maturity. | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Ramasubbaiah & Lal 1976) | Yes NSW, Vic., Tas., WA (APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
[Agromyzidae] Vegetable leaf miner |
Yes (CABI 2022; Firake et al. 2018) | Yes, Under official control (National). Restricted distribution and regulated in Qld (QDAF 2023). | No. Liriomyza sativae is a leaf miner that feeds primarily on the leaves of a host plant (CABI 2022; QDAF 2023). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Pal, Maji & Mondal 2013) | Yes, Under official control (National) (IPPC 2021). Present with restricted distribution in Qld and WA (Business Queensland 2021; DPIRD 2021). | No. Liriomyza trifolii is a leaf miner, primarily feeding on leaves of host plants, allowing possible secondary fungal and viral infections (Hore, Chakraborty & Banerjee 2017). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
[Agromyzidae] Okra stemfly; Okra petiole maggot |
Yes (Kanwar 2017) | No records found | No. Melanagromyza hibisci damages the petiole of okra plants, infesting stems and feeding on the pith inside the stem (Kanwar 2017). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (TNAU-NAIP 2020) | No records found | No. Melanagromyza obtusa is reported as a minor pest of okra (TNAU-NAIP 2020). The maggot of M. obtusa bores through the stem tissue, resulting in wilting and death of the affected plants or branches (Venugopal & Venkataramani 1954). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Bactrocera cucurbitae (Coquillett, 1899) [Tephritidae] |
Yes (Kumagai, Tsuchiya & Katsumata 1996; Sarada et al. 2020) | No records found | Yes. Zeugodacus cucurbitae has been reported to infest okra fruit (McQuate, Liquido & Nakamichi 2017; Wong et al. 1989). | Yes. Okra fruit will be distributed across Australia for sale and could potentially carry eggs and larvae. Immature stages that are present in imported okra could pupate and develop into adults and disperse to new hosts available in Australia. | Yes. Zeugodacus cucurbitae has the potential to establish and spread in Australia, as suitable hosts and environments are available. It has a wide range of hosts and is found across Asia (CABI 2022; Dhillon et al. 2005; Kumagai, Tsuchiya & Katsumata 1996). Its hosts and geographic distribution suggest that it could establish and spread in Australia. | Yes (EP) | ||||||||||||
| Hemiptera | ||||||||||||||||||
Synonym(s): Acrosternum hilare (Say, 1832) [Pentatomidae] |
Yes (Pal, Maji & Mondal 2013) | No records found | No. Chinavia hilaris is a member of the family Pentatomidae, an external feeder with nymphs and adults sucking sap from fruit of the host plant (Gomez & Mizell 2013). Chinavia hilaris is unlikely to be present in export quality okra as they characteristically drop from their hosts when disturbed, or fly away (Alcock 1971). Harvest and packing house practices will likely remove C. hilaris from the pathway. | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Prathapan 1996) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022) and Tas (DPIPWE Tasmania 2021). Present in NT, Qld (APPD 2022; DJPR 2019; Lambkin 1999). Aleurodicus dispersus is a suspected vector of at least 25 plant viruses (Banjo 2010). Therefore, this species is a potential regulated article for Australia. | No. This species is a phloem feeder and females lay eggs on the underside of leaves. Adults superficially feed externally on fruit (Banjo 2010; CABI 2022; Sathe & Gangate Ujjwala 2015). Adult whiteflies are very active and are unlikely to remain on the fruit when disturbed during harvesting and packing house practices. |
Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
| Yes (TNAU-NAIP 2020) | No records found. Leafhoppers can act as vectors for phytoplasmas in the 16SrI (B) group (Lee, Gundersen-Rindal & Bertaccini 1998; Lee et al. 2004b). Therefore, this pest is a potential regulated article for Australia. | No. The leafhopper Amrasca biguttula biguttula is associated with okra leaves (CABI 2022; Chandio et al. 2017). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Cerococcus hibisci Green 1908 [Cerococcidae] |
Yes (García Morales et al. 2022) | No records found | No. Antecerococcus indicus is a pest of okra (García Morales et al. 2022), but is only reported to feed on leaves and branches of host plants (Pushpaveni, Rao & Rao 1974). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Verma & Dinabandhoo 2005) | Yes. Qld, NSW, SA, Vic., Tas., NT, WA (APPD 2022; Dao et al. 2017; Government of Western Australia 2022; Naumann 1993) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Aphis craccivora Koch, 1854 [Aphididae] |
Yes (Singh et al. 1999) | Yes. Qld, NSW, SA, Vic., Tas., NT, WA (APPD 2022; Government of Western Australia 2022; Gutierrez et al. 1974) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (CABI 2022; DPP 2007) | No records found | No. Nymphs and adults of Aphis spp. feed externally on leaves by sucking plant sap (Kedar, Kumerang & Thodsare 2013). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Aphis gossypii Glover, 1877 [Aphididae] |
Yes (Singh et al. 1999) | Yes. Qld, NSW, SA, Vic., Tas., NT, WA (APPD 2022; Government of Western Australia 2022; Naumann 1993) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Tobacco whitefly |
Yes (Balikai, Kotikal & Prasanna 2009). There are at least five Bemisia tabaci species present in India, consisting of Asia I, Asia II-5, Asia II-7, Asia II-8 and MEAM1 (Chowda-Reddy et al. 2012). | Yes, but only some members of the complex. At least three species (AUS1, AUS II and MEAM 1) are known to be present in Australia, but most species in the complex remain absent from Australia. |
No. The Bemisia tabaci species complex is a phloem feeder and females lay eggs on the underside of leaves (Kedar, Kumerang & Thodsare 2013; Li et al. 2011; TNAU-NAIP 2020). Adult whiteflies are very active and are unlikely to remain on the fruit when disturbed during harvesting and packing house practices. | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (García Morales et al. 2022; Konar & Roy 2008) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in NSW, Qld (APPD 2022). | No. Ceroplastes floridensis primarily attack stems, leaves and branches of host plants (Drees, Reinert & Williams 2011). Direct damage is caused by scales feeding on cellular fluid in leaves. Excessive consumption of fluid results in secretion of honeydew, which enables fungi to develop on leaf surfaces (Drees, Reinert & Williams 2011). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Dysdercus cingulatus (Fabricius, 1775) [Pyrrhocoridae] |
Yes (Nair et al. 2017) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in NSW, NT, Qld, SA (APPD 2022; Naumann 1993). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
[Pyrrhocoridae] Red cotton bug |
Yes (Dhamdhere, Bahadur & Misra 1985) | No records found | No. Dysdercus koenigii feed on seeds inside of the host fruit using their stylus to pierce through the outer wall of the fruit (Shah 2014). Adults and nymphs are highly mobile and are unlikely to remain on the fruit when disturbed during harvesting and packing house practices. | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Pal, Maji & Mondal 2013) | Yes. NSW, NT, Qld, WA (ALA 2023; APPD 2022; CABI 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Pentatoma halys Stål, 1855 [Pentatomidae] |
Yes (Rout et al. 2018) | No records found | No. Halyomorpha halys adults suck sap externally from the fruit of okra (Kuhar et al. 2012; Zobel, Hooks & Dively 2016). Pentatomid bugs are not likely to be associated with the fruit because they characteristically drop from their hosts or fly away when disturbed (Alcock 1971). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Varshney 1992) | No records found | No. Okra is reported to be a host of Icerya formicarum (Varshney 1992). However, Icerya spp. primarily feed on the stems and the lower side of leaves of its host plants (Watson & Malumphy 2004). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Jacobiasca lybica de (Bergevin & Zanon, 1922) Synonym(s): Chlorita lybica Bergevin & Zanon, 1922 |
Yes (Sohi, Shinger & Mann 1988) | No records found. Leafhoppers can act as vectors for phytoplasmas in the 16SrI (B) group (Lee, Gundersen-Rindal & Bertaccini 1998; Lee et al. 2004b). This pest is therefore also a potential regulated article for Australia. | No. Jacobiasca lybica feeds on leaves of okra plants (Hendawy, El-Fakharany & Hegazy 2017). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Largidae] Giant red bug |
Yes (Government of India 2007) | No records found | No. Nymphs and adults of L. grandis feed on fruit, stems and leaves of host plants (Joshi & Khan 1990). Eggs of L. grandis are deposited in soil and are not associated with okra fruit (Joshi & Khan 1990). Harvesting and packing house practices will likely remove the large sized, externally feeding L. grandis from the pathway. | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Nagrare, Kumar & Dharajothi 2014) | Yes. NT, Qld, Vic., SA, WA (ALA 2023; APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Myzus persicae (Sulzer, 1776) [Aphididae] |
Yes (Sharma & Rao 2012) | Yes. NT, Qld, SA, Vic., NSW, Tas., WA (CABI 2022; Government of Western Australia 2022; Vorburger, Lancaster & Sunnucks 2003) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Green stink bug; Green vegetable bug |
Yes (Government of India 2007) | Yes. Qld, NSW, NT, Vic., SA, Tas., WA (APPD 2022; Coombs 2004; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Nipaecoccus viridis (Newstead, 1894) |
Yes (Varshney 1992) | Yes. NT, Qld, WA (APPD 2022; Bellis et al. 2004; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Lygaeidae] Dusky cotton bug; Cotton seed bug |
Yes (Kedar, Kumerang & Thodsare 2013; TNAU-NAIP 2020) | No records found | No. Oxycarenus hyalinipennis is an externally feeding polyphagous pest and nymphs and adults are reported to infest okra (Kedar, Kumerang & Thodsare 2013; Shah et al. 2016). However, harvesting and packing house practices would likely remove the externally feeding O. hyalinipennis from the pathway. | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Sakthivel et al. 2012) | No records found | Yes. Paracoccus marginatus is a pest of okra (Sakthivel et al. 2012). This species sucks the sap from various parts of the host plant, including the leaves, stems, flowers and fruit (Khan et al. 2014; Mani, Shivaraju & Shylesha 2012). Due to its small size, it is possible that an early stage of infestation on okra fruit may remain undetected and be present on the pathway. | Yes. Paracoccus marginatus has a wide host range including crop plants and ornamentals (Krishnan et al. 2016), and many hosts are available in Australia. Imported okra will be distributed throughout Australia via the wholesale and retail trade pathway. Mealybugs present on discarded okra fruit waste could potentially disperse to a new host within close proximity. | Yes. Assessed in the mealybug group PRA (DAWR 2019). | Yes. Assessed in the mealybug group PRA (DAWR 2019). | Yes (GP) | ||||||||||||
Synonym(s): Lecanium nigrum Nietner, 1861 [Coccidae] |
Yes (Ananda 2007; Balikai, Kotikal & Prasanna 2009) | Yes. Qld, NSW, NT, Vic., SA, WA (Government of Western Australia 2022; Lin et al. 2017a; Lin et al. 2017b; Naumann 1993) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Ben-Dov 1994; CABI 2022; Shylesha & Joshi 2012) | No records found | Yes. Phenacoccus madeirensis is reported to be a pest of okra (Ben-Dov 1994). In India, P. madeirensis heavily infests all above-ground parts of Malvaceae host plants, causing severe damage by feeding externally on leaves, stems, flowers and fruit (Shylesha & Joshi 2012). Due to its smaller size, it is possible that P. madeirensis on okra fruit may remain undetected and be present on the pathway. | Yes. Phenacoccus madeirensis has a wide host range including crop plants and ornamentals (CABI 2022), and many hosts are available in Australia. Imported okra will be distributed throughout Australia via the wholesale and retail trade pathway. Mealybugs present on discarded okra fruit waste could potentially disperse to a new host within close proximity. | Yes. Assessed in the mealybug group PRA (DAWR 2019). | Yes. Assessed in the mealybug group PRA (DAWR 2019). | Yes (GP) | ||||||||||||
Phenacoccus solenopsis (Tinsley 1898) [Pseudococcidae] |
Yes (Kedar, Kumerang & Thodsare 2013) | Yes. Qld, NT, WA (APPD 2022; DAF 2013; Government of Western Australia 2023) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Parveen et al. 2015) | Yes. NSW, NT, SA, Qld, Vic., ACT, WA (ALA 2023; APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Pinnaspis strachani (Cooley, 1899) Synonym(s): Hemichionaspis strachani, 1899 |
Yes (Suresh & Mohanasundaram 1996) | Yes. NT, Qld, WA (APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Diaspididae] Mulberry scale; White peach scale |
Yes (Nakahara 1982) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld, NSW (CABI 2022). | Yes. Pseudaulacaspis pentagona has a wide host range including crop plants and ornamentals (Malumphy et al. 2016), and many hosts are available in Australia. Imported okra will be distributed throughout WA via the wholesale and retail trade pathway. Scales present on discarded okra fruit waste could potentially disperse to a new host within close proximity. | Yes. Assessed in the scale group PRA (DAWE 2021). | Yes. Assessed in the scale group PRA (DAWE 2021). | Yes (GP, WA) | ||||||||||||
[Asterolecaniidae] Oleander pit scale |
Yes (García Morales et al. 2022) | No records found | No. Russellaspis pustulans pustulans is usually restricted to branches and stems, inducing galls around feeding sites (Gullan, Miller & Cook 2005). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Konar & Roy 2008; TNAU-NAIP 2020) | Yes NSW, Qld, NT, SA, Vic., Tas., WA (APPD 2022; Ben-Dov 1993; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Saissetia miranda (Cockerell & Parrott in Cockerell, 1899) Synonym(s): Lecanium miranda Cockerell & Parrot, 1899 |
Yes (Varshney 1992) | Yes. Qld, NT, WA (APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Diaspidae] Rufous scale; West Indian red scale |
Yes (Mamet 1958b) | Not present, Selenaspidus articulatus is listed as present in (Mamet 1958a), however it is considered absent due to the unreliability of the record. | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
[Aleyrodidae] Greenhouse whitefly |
Yes (Roopa et al. 2012) | Yes. Qld, NSW, SA, NT, Vic., Tas., WA (APPD 2022; Gambley et al. 2010; Government of Western Australia 2022). Trialeurodes vaporariorum is a vector of Tomato leaf curl New Delhi virus (ToLCNDV) (Fiallo-Olivé et al. 2020), which is a quarantine pest for Australia. Therefore, T. vaporariorum is a regulated article for Australia. | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
| Lepidoptera | ||||||||||||||||||
[Noctuidae] Black cutworm |
Yes (Government of India 2007) | Yes. Qld, NSW, NT, SA, Tas., WA (APPD 2022; Common 1990; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Government of India 2007) | No records found | No. Agrotis segetum adults are highly polyphagous and reported to feed on stems or leaves (Moir et al. 2007). Eggs of A. segetum are laid in soil (Moir et al. 2007). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Amsacta moorei (Butler, 1876) [Arctiidae] |
Yes (Netam, Ganguli & Dubey 2007) | No records found | No. Amsacta moorei larvae feed on leaves of the host plant (CABI 2022). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Yellow scallop moth; Abutilon moth |
Yes (Vishakantaiah & Govindan 1975) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in NSW (Gurney 1924). | No. Anomis erosa has only been reported as a leaf defoliator (Chittenden 1913). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Anomis flava (Fabricius, 1775) |
Yes (Government of India 2007; Nair et al. 2017; TNAU-NAIP 2020) | Yes. Qld, NSW, NT, WA (ALA 2023; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Noctuidae] |
Yes (Nair et al. 2017) | Yes, Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld (APPD 2022). | No. Anomis fulvida has only been reported as a minor pest of okra leaves (Nair et al. 2017). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Anomis sabulifera (Guenée, 1852) |
Yes (Majumder et al. 2018) | No records found | No. Anomis spp. larvae are leaf feeders and pupate inside folded leaves (Kravchenko et al. 2014; Nair et al. 2017; TNAU-NAIP 2020). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Tortricidae] Soyabean leafroller |
Yes (Gilligan, Baixeras & Brown 2018; Pathania et al. 2020; Sharma et al. 2008) | No records found | No. Archips micaceana is reported as a defoliator (Sottikul 1989). Other Archips spp. are primarily leaf or stem feeders (Brunner 1993; Razowski 1977). The eggs of Archips spp. are laid on the leaf surface, or on the soil surface (Razowski 1977). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Pathania et al. 2020; Robinson et al. 2022) | No records found | No. Archips spp. are primarily leaf feeders (Brunner 1993; Razowski 1977). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Noctua signata Fabricius, 1775; Plusia diminuta (Walker, 1865); Plusia signata (Holloway 1976) [Noctuidae] |
Yes (Rao, Thontadarya & Rangadhamaiah 1979) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld (Herbison-Evans & Crossley 2022). | No. Argyrogramma signata has only been reported as a foliage feeder (Herbison-Evans & Crossley 2022). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (CABI 2022; Government of India 2007) | Yes. NSW, NT, Qld, SA, Tas., Vic., WA (APPD 2022; CABI 2022; Common 1990; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Crocidosema plebejana (Zeller, 1847) [Tortricidae] |
Yes (Government of India 2007; Pathania et al. 2020) | Yes. Qld, NSW, NT, SA, Vic., Tas., WA (APPD 2022; CABI 2022; Common 1990; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
African moth; Safflower caterpillar |
Yes (Robinson et al. 2022; Smetacek 2008) | No records found | No. Candica capensis larvae are reported to feed on leaves and stems of the host plants (Chakravarthy & Sridhara 2016). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Corcyra cephalonica (Stainton, 1866) |
Yes (Kaur 2020; Robinson et al. 2022) | Yes. NSW, NT, Qld, Vic., WA (APPD 2022; CABI 2022; Common 1990; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Saturnia trifenestrata (Helfer, 1837); Cricula andrei (Holloway, 1976) [Saturniidae] |
Yes (CABI 2022; Robinson et al. 2022; Tikader, Vijayan & Saratchandra 2014) | No records found | No. Cricula trifenestrata larvae usually feed on leaves and stems of the host plants (Plantwise 2023). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Pillai & Kumar 2020) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld (APPD 2022). | No. Delias eucharis is reported to feed on foliage (Naidu, Reddy & Ramana 2011). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Dudua aprobola (Meyrick, 1886) Synonym(s): Eccopsis aprobola (Meyrick, 1886) |
Yes (Pathania et al. 2020; Robinson et al. 2022) | Yes. Qld, NT, NSW, WA (APPD 2022; Nielsen, Edwards & Rangsi 1996; Zborowski & Edwards 2007) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Noctuidae] Spiny bollworm; Shoot and fruit borer |
Yes (Smetacek 2008) | No records found | No. Earias biplaga is a major pest of cotton; but, reported on okra. It feeds on the terminal shoots and fruit of host plants (Hill 2008; Munthali & Tshegofatso 2013). Earias spp. larvae bore into fruit, leaving noticeable bore holes filled with frass, often deforming fruit and causing premature fruit drop (Butani & Jotwani 1984; Hill 2008; Kedar, Kumerang & Thodsare 2013; Vennila et al. 2007). Eggs of E. biplaga are 0.5 mm, blue/green and laid indiscriminately over the whole plant (Entwistle 1969; Hill 2008). First instar larvae are 0.23 mm wide and white, darkening to a pale brown as they mature (Entwistle 1969; Hill 2008). Size/colour of the eggs and larvae, and symptoms caused, would make the pest unlikely to be present in commercially prepared export quality okra from India. | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Dhawan & Sidhu 1984; Muddasar & Venkateshalu 2018) | No records found | mature (Dadasaheb 2007; Hill 2008). The size and colour of the eggs/larvae, and damage caused, would make the pest unlikely to be present on export quality okra fruit. |
Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Earias insulana (Boisduval, 1833) |
Yes (Konar & Rai 1990) | No records found | The size and colour of the eggs/ larvae, and the damage caused, would make the pest unlikely to be present on export quality okra fruit. |
Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Earias vittella (Fabricius, 1794) |
Yes (Kedar, Kumerang & Thodsare 2013) | Yes. Qld, NT, NSW, WA (ALA 2023; APPD 2022; Common 1990; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Artaxa fraterna (Moore, 1883) [Lymantriidae] |
Yes (Manoharan, Chockalingam & Noorjahan 1982; Venkatesha, Gopinath & Chandramohan 1992) | No records found | No. Euproctis fraterna is a leaf feeder of okra (Manoharan, Chockalingam & Noorjahan 1982; Venkatesha, Gopinath & Chandramohan 1992). Larvae feed on the epidermal tissues of leaves of host plants by scraping the chlorophyll content of leaves, resulting in the skeletonization of leaves (Nizamani et al. 2016). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Cotton leaf roller; Hibiscus leafroller |
Yes (Chakraborty, Kumar & Rajadurai 2014; TNAU-NAIP 2020) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld, NSW, NT (ALA 2023; APPD 2022; PestNet 2022) | No. Haritalodes derogata larvae primarily feed on the leaves and stems of the host plants (TNAU-NAIP 2020). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Helcystogramma hibisci (Stainton, 1859) |
Yes (Ponomarenko 1997; Sharma et al. 2008) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld (ALA 2023; APPD 2022; Common 1990) | No. Helcystogramma hibisci has only been associated with the leaves of okra (Butani & Jotwani 1984). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Noctua armigera Hübner, 1808; Heliothis armigera (Hübner, 1808) [Noctuidae] |
Yes (TNAU-NAIP 2020) | Yes. Qld, NSW, SA, NT, Vic., Tas., WA (Government of Western Australia 2022; Naumann 1993) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
American cotton bollworm; Corn earworm moth |
No. Helicoverpa zea was reported to be present in India in a preliminary study by Sharma and Rao (2012), but likely misidentified. Helicoverpa zea is distributed in North America and South America (CABI 2022). There is no further evidence supporting the presence of H. zea in India, or Asia in general. | No records found | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Homona coffearia Nietner, 1861 |
Yes (Pathania et al. 2020; Robinson et al. 2022) | No records found | No. Homona coffearia is leaf roller and the larvae make shelters by fastening 2 or more leaves together with silk and feeding inside the leaf (Cranham & Danthanarayana 1971). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Papilio misippus Linnaeus, 1764 [Nymphalidae] |
Yes (Robinson et al. 2022; Varshney & Smetacek 2015) | Yes. Qld, NT, NSW, WA (ALA 2023; APPD 2022; Braby 2000; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Eggplant fruit and shoot borer |
Yes (Dixit & Awasthi 2017) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld (APPD 2022). | No. Leucinodes orbonalis has been reported to be almost entirely restricted to Solanum spp. or Solanaceae (Hayden et al. 2013; Herbison-Evans & Crossley 2022; Mainali 2014; Robinson et al. 2022) and is reported to be a major pest of eggplant (Ardez, Sumalde & Taylo 2008; Dixit & Awasthi 2017). However, Leucinodes orbonalis has been intercepted on okra from Ghana to the United States (Boateng et al. 2005). According to a choice assay between eggplant and a number of other plants (including okra), L. orbonalis oviposits solely on eggplant and the pest was unable to complete its life cycle on okra (Ardez, Sumalde & Taylo 2008). |
Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Cowpea pod borer; Bean pod borer; Mung moth |
Yes (Rathee & Dalal 2018) | Yes. NT, NSW, Qld, WA (ALA 2023; APPD 2022; Business Queensland 2018; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Ochropleura flammatra (Denis & Schiffermüller, 1775) |
Yes (Gupta 1990; Singh & Misra 1988) | No records found | There is no evidence available for the association between this pest and okra fruit in India. |
Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Pardoxia graellsii (Feisthamel, 1837) |
Yes (De Prins & De Prins 2022) | No records found | No. Pardoxia graellsii is reported as a major pest of okra in India, feeding on leaves and occasionally defoliating whole plants (Dwomoh & Boakye 2003). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Depressaria gossypiella (Saunders, 1844) [Gelechiidae] |
Yes (Murthy, Nagaraj & Prabhuraj 2018) | Yes. Qld, NT, SA, WA (ALA 2023; APPD 2022; Common 1990; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Diamondback moth |
Yes (Pandey et al. 2006) | Yes. NSW, Qld, NT, Vic., Tas., WA (APPD 2022; Common 1990; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Somena scintillans (Walker, 1855) |
Yes (Gupta, Tara & Pathania 2013; Robinson et al. 2022) | No records found | No. Okra is reported to be a host of S. scintillans; however, it is primarily a leaf feeder (Robinson et al. 2022; Sharma & Ramamurthy 2009). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Diacrisia obliqua (Walker, 1855) [Arctiidae] |
Yes (Nair et al. 2017) | No records found | No. Spilosoma obliqua is a leaf feeder (Nair et al. 2017). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Beet armyworm; Lesser armyworm |
Yes (Pathan et al. 2018; Robinson et al. 2022) | Yes. ACT, NSW, NT, Qld, SA, Tas., Vic., WA (APPD 2022; Common 1990; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Spodoptera frugiperda (Smith and Abbot, 1797) |
Yes (Mahadeva Swamy et al. 2018) | Yes. NT, Qld, WA, Tas. (Biosecurity Tasmania 2021; Government of Western Australia 2022; IPPC 2020) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Hadena littoralis Boisduval; Noctua gossypii [Noctuidae] |
Yes (Sivasankaran et al. 2012) | No records found | No. Spodoptera littoralis is only known as a leaf feeder of okra (Obeng-Ofori & Sackey 2003). Spodoptera littoralis has not been recorded attacking okra fruit (Gerson & Applebaum 2022). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Taro caterpillar; Cluster caterpillar |
Yes (CABI 2022; Chakraborty, Kumar & Rajadurai 2014) | Yes. Qld, NSW, NT, Vic., Tas., WA (APPD 2022; Government of Western Australia 2022; Naumann 1993) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Trichoplusia ni (Hübner, 1803) |
Yes (Jagtap, Shetgar & Nalwandikar 2007) | No records found | No. Trichoplusia ni is a leaf feeder of okra (Capinera 2011). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Acontia nitidula (Fabricius, 1787) [Noctuidae] |
Yes (Kannan & Uthamasamy 2006; TNAU-NAIP 2020) | No records found | No. Tarache nitidula is a pest of okra in India and is a leaf miner (Kannan & Uthamasamy 2006). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Looper |
Yes (Singh, Singh & Singh 1973) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in NSW, Qld (Balciunas, Burrows & Edwards 1993). | No. Thalassodes quadraria has been reared on okra fruit in no-choice assays in a laboratory study; however, there are no records of this pest attacking okra fruit in the field and it is regarded as an external leaf feeder (Muhamed, Kumari & Kurien 2018; Singh, Singh & Singh 1973). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Xanthodes intersepta (Guenée, 1852) |
Yes (Singh & Joshi 2003) | No records found | No. Xanthodes spp. are reported as minor pests of okra and are primarily leaf feeding pests (Nair et al. 2017; Sahayaraj 2015). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Noctua albago (Fabricius, 1794); Xanthodes malvae (Esper, 1805) [Noctuidae] |
Yes (Nair et al. 2017) | Yes. Qld, WA (APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Transverse moth |
Yes (Nair et al. 2017) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in NSW, Qld (APPD 2022; Common 1990). | No. Xanthodes transversa larvae are reported to feed primarily on leaves and tender stems of host plants (Nair et al. 2017). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Zeuzera coffeae Nietner, 1861 |
Yes (Government of India 2007; Remadevi & Raja 1998) | No records found | No. Zeuzera coffeae is a stem borer in host plants (Remadevi & Raja 1998). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Orthoptera | ||||||||||||||||||
Synonym(s): Gryllus axillaris (Thunberg, 1815) Catantops axillaris (Jago, 1984) [Acrididae] |
Yes (Kumar & Usmani 2014) | No records found | No. Diabolocatantops axillaris is reported as a minor pest of okra (Anderson 1964). Nymphs and adults of D. axillaris have only been reported to feed on the leaves and flowers of okra (Anderson 1964). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Vedham, Kolatkar & Muralirangan 2002) | No records found | No. Diabolocatantops pinguis is reported to survive on okra in the absence of preferred hosts (Vedham, Kolatkar & Muralirangan 2002). Diabolocatantops pinguis is polyphagous and is primarily known to feed on the leaves of the host plant (Ayyasamy & Regupathy 2013). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Gryllus pictus (Fabricius, 1775); Poekilocerus pictus (Fabricius, 1775) [Acrididae] |
Yes (Thara et al. 2019; TNAU-NAIP 2020) | No records found | No. Poecilocerus pictus is a minor pest of okra (TNAU-NAIP 2020). It primarily feeds on the leaves and stem of the host plant (Sharma 1991). Eggs of P. pictus are deposited in soil and the pest is not known to be associated with okra fruit (Sultana et al. 2015). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Srinivasan & Prabakar 2013) | No records found | No. Oxya fuscovittata is a minor pest of okra (Srinivasan & Prabakar 2013). Oxya fuscovittata is most often associated with leaf feeding and there is no evidence to suggest that O. fuscovittata is associated with okra fruit (Srinivasan & Prabakar 2013). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Oxya japonica (Thunberg, 1815) Synonym(s): Gryllus japonicus Thunberg, 1815 |
Yes (TNAU-NAIP 2020) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld (ALA 2023; APPD 2022) | No. Oxya japonica is a minor pest of okra (TNAU-NAIP 2020). Oxya japonica is often associated with the leaves of grasses (Tajamul & Ahmad 2016). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Thysanoptera | ||||||||||||||||||
[Thripidae] Eurasian flower thrips |
Yes (CABI 2022; Rachana et al. 2020) | No records found | Yes. Frankliniella intonsa has a wide host range including crop plants and ornamentals (Miyazaki & Kudo 1988), and many hosts are available in Australia. Imported okra will be distributed throughout Australia via the wholesale and retail trade pathway. Thrips present on discarded okra fruit waste could potentially disperse to a new host within close proximity. | Yes. Assessed in the thrips Group PRA (DAWR 2017). | Yes. Assessed in the thrips Group PRA (DAWR 2017). | Yes (GP) | ||||||||||||
[Thripidae] Chilli thrips |
Yes (Balikai, Kotikal & Prasanna 2009; CABI 2022; Tyagi & Kumar 2014) | Scirtothrips dorsalis has a wide host range including crop plants and ornamentals (CABI 2022), and many hosts are available in Australia. Imported okra will be distributed throughout Australia via the wholesale and retail trade pathway. Thrips present on discarded okra fruit waste could potentially disperse to a new host within close proximity. | Not applicable to vector. However, the emerging quarantine orthotospoviruses vectored by this thrips have potential for establishment and spread (DAWR 2017). | Not applicable to vector. However, the emerging quarantine orthotospoviruses vectored by this thrips have potential for consequences (DAWR 2017). | Yes (GP, RA) | |||||||||||||
Thrips palmi (Karny, 1925) Synonym(s): Thrips clarus (Moulton, 1928); Thrips gossypicola (Priesner, 1939) |
Yes (Sushil et al. 2020; Tyagi & Kumar 2014) | Yes, Under official control (Regional) for SA and WA (Government of Western Australia 2022; PIRSA 2019). Present in NSW, NT, Qld, WA (APPD 2022; Government of Western Australia 2022). | Yes. Thrips palmi is a polyphagous species that attacks many hosts in Cucurbitaceae, Solanaceae and Fabaceae (CABI 2022; Young & Zhang 1998), and many hosts are available in Australia. Imported okra will be distributed throughout WA and SA via the wholesale and retail trade pathway. Thrips present on discarded okra fruit waste could potentially disperse to a new host within close proximity. | Yes. Assessed in the thrips Group PRA (DAWR 2017). | Yes. Assessed in the thrips Group PRA (DAWR 2017). | Yes (GP, SA, WA) | ||||||||||||
| Trombidiformes | ||||||||||||||||||
Aculops lycopersici (Tryon, 1917) |
Yes (Kashyap, Sharma & Sood 2015; Kumar, Raghuraman & Singh 2015) | Yes. NSW, Qld, SA, Vic., Tas., NT, WA (ALA 2023; APPD 2022; CABI 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
(Banks, 1904) Synonym(s): Tenuipalpus californicus Banks, 1904; Brevipalpus australis (Baker, 1949) |
Yes (Mitra, Acharya & Ghosh 2018; Plantwise 2023) | Yes. NSW, SA, Vic., Tas., NT, WA (ALA 2023; APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Tenuipalpidae] Scarlet tea mite |
Yes (Gupta 1985) | Yes. NSW, Vic., Qld, NT, WA (APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Beard et al. 2015) | Yes. Qld, NT, WA (Beard et al. 2015) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Eutetranychus orientalis (Klein, 1936) Synonym(s): Anychus orientalis Klein, 1936; Eutetranychus anneckei (Meyer, 1974) |
Yes (Balikai, Kotikal & Prasanna 2009; Kumawat & Singh 2002) | Yes. Qld, NT, WA (ALA 2023; Government of Western Australia 2022; Walter, Halliday & Smith 1995) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Tetranychidae] Spider mite |
Yes (CABI 2022; Government of India 2007; Jaydeb, Mukherjee & Sarkar 1996) | Yes. Under official control (Regional) for WA (Government of Western Australia 2022). Present in Qld (Halliday 2000). | No. Oligonychus biharensis is only known to feed on the leaves of host plants (Kaimal & Ramani 2011) | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Ghosh 2004) | No records found | No. Oligonychus gossypii primarily feeds on the leaves and stems of okra (Boateng et al. 2005; Migeon & Dorkeld 2022) | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Hemitarsonemus latus (Banks, 1904); Tarsonemus latus Banks, 1904 [Tarsonemidae] |
Yes (Grewal 1992; Gupta 1985; Prasad & Singh 2011) | Yes. NSW, Qld, SA, Vic., NT, WA (ALA 2023; APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Red spider mite; Bean spider mite |
Yes (Kumar, Raghuraman & Singh 2015) | Yes. Qld, NSW, NT, Vic., WA, SA (ALA 2023; APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Tetranychus macfarlanei Baker & Pritchard, 1960 |
Yes (Migeon & Dorkeld 2022; Prasad & Singh 2011) | No records found | Yes. Tetranychus macfarlanei is a major pest of okra in India (Jeppson, Keifer & Baker 1975; Kumar, Raghuraman & Singh 2015; Rajgopal & Srinivasa 2017). Okra red spider mites usually feed on leaves, causing various symptoms like yellowing, bronzing and causing the formation of chlorotic spots on the feeding surface of leaves (Satyagopal et al. 2014). In the case of heavy infestation, leaves wither and dry and flower and fruit formation is affected (Satyagopal et al. 2014). | Yes. Okra fruit will be distributed across Australia for sale and could potentially carry mite nymphs and/or adults. Tetranychus macfarlanei is polyphagous and suitable hosts may be available within the proximity, especially in rural/regional Australia. Spider mites primarily disperse by crawling. Although less likely, it is possible that spider mites present on discarded okra fruit waste could potentially find suitable hosts within close proximity (Kennedy & Smitley 1985). | Yes. Tetranychus macfarlanei has the potential to establish and spread in Australia as suitable hosts and environments are available. This species has established in areas with a wide range of climatic conditions (Bolland, Gutierrez & Flechtmann 1998; Jeppson, Keifer & Baker 1975; Zeity, Srinivasa & Gowda 2017). Tetranychus macfarlanei is polyphagous, feeding on several host plants (Bolland, Gutierrez & Flechtmann 1998; Zeity, Srinivasa & Gowda 2017). Some of these hosts are widespread in Australia. | Yes. Tetranychus macfarlanei has caused serious damage to okra, eggplant, pumpkin and cucumber (Jeppson, Keifer & Baker 1975). In India, it is a serious pest of okra, cotton, soybean, eggplant and other cucurbits (Latha et al. 2019; Rajgopal & Srinivasa 2017; Satish et al. 2018; Zeity, Srinivasa & Gowda 2017). In India, soybean fields infested with red spider mites can cause 40-60% yield reduction (Satish et al. 2018). It is also an important pest of many agricultural crops in Bangladesh (Ali, Naif & Huang 2011). | Yes | |||||||||||
Mariana mite |
Yes (Migeon & Dorkeld 2022; Zeity, Srinivasa & Gowda 2016) | Yes. Qld, NT, WA (APPD 2022; CABI 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Tetranychus neocaledonicus André, 1933 |
Yes (Rajgopal & Srinivasa 2017; Singh & Chauhan 2019) | Yes. NT, Qld, WA (ALA 2023; APPD 2022; Government of Western Australia 2022; Seeman & Beard 2011) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Tetranychidae] |
Yes (Gupta & Bose 2017; Migeon & Dorkeld 2022) | No records found | No. Okra is reported as a host of Tetranychus puschelii (Migeon & Dorkeld 2022) in Africa. However, there is no further evidence available for the association between this pest and okra fruit in India, or other countries. | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Tetranychus truncatus Ehara, 1956 |
Yes (Bachhar et al. 2019; Migeon & Dorkeld 2022) | No records found | Yes. Tetranychus truncatus is a serious pest of okra in Kerala, India (Bachhar et al. 2019). Tetranychus truncatus usually feeds and produces webbing on the lower surface of the leaf. In cases of heavy infestation, Tetranychus spp. colonies cover whole plants, including the flowers and fruit (Satyagopal et al. 2014). | Yes. Okra fruit will be distributed across Australia for sale and could potentially carry mite nymphs and/or adults. Tetranychus truncatus is polyphagous and suitable hosts may be available in close proximity, especially in rural/regional Australia. Spider mites primarily disperse by crawling. Although less likely, it is possible that spider mites present on discarded okra fruit waste could potentially find suitable hosts within close proximity (Kennedy & Smitley 1985). | Yes. Tetranychus truncatus has the potential to establish and spread in Australia as suitable hosts and environments are available. This species has established in areas with a wide range of climatic conditions (Bolland, Gutierrez & Flechtmann 1998; Migeon & Dorkeld 2022). This species is polyphagous, feeding on several host plants (Bolland, Gutierrez & Flechtmann 1998; Migeon & Dorkeld 2022). Some of these hosts are widespread in Australia. | Yes. Tetranychus truncatus has the potential for economic consequences in Australia. Tetranychus truncatus is highly polyphagous, causing damage to economically important crops, including cotton, jute, maize, papaya and many vegetable crops (Jin et al. 2018; Migeon & Dorkeld 2022; Vacante 2016). Tetranychus truncatus can reduce crop yield through feeding and from the large amounts of webbing (Ullah, Gotoh & Lim 2014). | Yes | |||||||||||
[Tetranychidae] Strawberry spider mite |
Yes (Gupta & Gupta 1994; Migeon & Dorkeld 2022) | No records found | No. Tetranychus turkestani has only been reported feeding on the leaves of host plants (Carey & Bradley 1982). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (DPP 2007; Gupta 1985; Kumar, Raghuraman & Singh 2015; Kumaran, Douressamy & Ramaraju 2007) | Yes. NSW, NT, Qld, SA, Tas., Vic., WA (APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
| BACTERIA | ||||||||||||||||||
Bacillus subtilis (Ehrenberg 1835) Cohn 1872 Synonym(s): Vibrio subtilis Ehrenberg 1835 |
Yes (Rao et al. 2014) | Yes. NSW, Qld, SA (APPD 2022; Broadbent, Baker & Waterworth 1971) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Savitri et al. 2017) | Yes. NSW (Elhalis, Cox & Zhao 2020) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Pectobacterium carotovorum (Jones 1901) Waldee 1945 (Approved Lists 1980) emend. Portier et al. 2019 Synonym(s): Bacillus carotovorus Jones 1901; Bacterium carotovorum (Jones 1901) Lehmann and Neumann 1927; Erwinia carotovora (Jones 1901) Bergey et al. 1923 |
Yes (Maisuria & Nerurkar 2013; Plantwise 2023) | Yes. NSW, Qld, SA, Vic., WA (APPD 2022; Government of Western Australia 2022; Waleron, Waleron & Lojkowska 2013) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Bacillales: Bacillaceae] |
Yes (Baliah & Muthulakshmi 2017) | Yes. NSW, WA (Fluidquip Australia 2009; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Pseudomonas cichorii (Swingle 1925) Stapp 1928 |
Yes (Babu et al. 2013) | Yes. NSW, Qld, NT, WA, Vic. (APPD 2022; Government of Western Australia 2022; Peters et al. 2004) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Pseudomonadales; Pseudomonadaceae] Bacterial canker |
Yes (Kumar 2019) | Yes. NSW, Qld, WA, SA, Vic., Tas., NT (APPD 2022; Government of Western Australia 2022; Peters et al. 2004) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Muthaiyan 2009) | No records found | No. Xanthomonas campestris pv. esculenti causes leaf blight in okra (Kumar 2019). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
| CHROMALVEOLATA | ||||||||||||||||||
Synonym(s): Phytophthora hydrophila Curzi 1927; Phytophthora mexicana Hotson & Hartge [Peronosporales: Peronosporaceae] |
Yes (Chowdappa 2017) | Yes. NSW, Qld, WA (APPD 2022; Government of Western Australia 2022; Weinert et al. 1998) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Root and stem rot |
Yes (Khare et al. 2016) | Yes. NSW, Qld, NT, Vic., WA (APPD 2022; Barber et al. 2013; Vawdrey 2001) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Phytophthora nicotianae Breda de Haan |
Yes (Chowdappa et al. 2016) | Yes. NSW, Qld, NT, WA, SA, Vic. (APPD 2022; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| FUNGI | ||||||||||||||||||
Synonym(s): Alternaria tenuis Nees, Torula alternata Fr.; Macrosporium fasciculatum Cooke & Ellis; Macrosporium erumpens Cooke; Macrosporium meliloti Peck; Macrosporium polytrichi Peck; Macrosporium seguierii Allesch [Pleosporales: Pleosporaceae] |
Yes (Dadabhau 2009) | Yes. NSW, Qld, NT, WA, SA, Vic., Tas. (APPD 2022; Le & Gregson 2019) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Hurule et al. 2019) | Yes. NSW, WA, Vic. (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Macrosporium hibiscinum Thuem [Pleosporales: Pleosporaceae] |
Yes (Khare et al. 2016) | No records found | No. Alternaria hibiscina is reported to cause leaf spot (Khare et al. 2016). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Pleosporales: Pleosporaceae] Fruit rot |
Yes (Khare et al. 2016) | Yes. NSW, WA (APPD 2022; Government of Western Australia 2022; Moslemi et al. 2017) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Varshney 1986) | Yes. ACT, NSW, Qld, Vic. (APPD 2022; Auld, Talbot & Radburn 1992) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Helminthosporium tenuissimum Nees & T. Nees : Fr.; Macrosporium tenuissimum (Nees & T. Nees : Fr.) Fr. [Pleosporales: Pleosporaceae] |
Yes (Vashisht & Chauhan 2016) | Yes. NSW, Qld, WA, SA, Vic., Tas. (APPD 2022; Government of Western Australia 2022; Harteveld, Akinsanmi & Drenth 2013) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Powdery mildew |
Yes (Gopalakrishnan & Valluvaparidasan 2009) | Yes. NSW, Qld, SA, Vic., Tas. (APPD 2022; Clare 1964) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Ascochyta abelmoschi Harter |
Yes (Sohi & Puttoo 1973) | Yes. Qld (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Eurotiales: Trichocomaceae] Aspergillus ear rot |
Yes (Kumkum, Sindhu & Shagufta 1989) | Yes. NSW, Qld, NT, WA, Vic. (APPD 2022; Geiser, Pitt & Taylor 1998; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Kumar et al. 2013b) | Yes. NSW, Qld, WA, Vic., Tas. (APPD 2022; Government of Western Australia 2022; Talbot et al. 2018) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Emericella nidulans (Eidam) Vuillemin; Diplostephanus nidulans (Eidam) Neveu-Lem [Eurotiales: Aspergillaceae] |
Yes (Yadav, Kushwaha & Jain 2020) | Yes. NSW, Vic. (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Kumkum, Sindhu & Shagufta 1989) | Yes. NSW, Qld, NT, WA, SA, Vic. (APPD 2022; Government of Western Australia 2022; Varga et al. 2007) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
[Eurotiales: Trichocomaceae] |
Yes (Prasad et al. 2000a) | Yes. Qld, Tas. (APPD 2022; Farr & Rossman 2020) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Aspergillus ustus (Bainier) Thom & Church |
Yes (Kulkarni & Chavan 2010) | Yes. Qld, Tas. (APPD 2022; Farr & Rossman 2020) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Atheliales: Atheliaceae] Sclerotium rot |
Yes (Mahadevakumar et al. 2016) | Yes. NSW, Qld, NT, WA, SA, Vic., Tas. (APPD 2022; Bhuiyan et al. 2019; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (CABI 2022; Shukla, Fatima & Kumari 2021) | Yes. NSW, Qld, SA, Vic., Tas., WA (APPD 2022; Government of Western Australia 2022; Harvey, Nehl & Aitken 2004) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Boeremia exigua (Desm.) Aveskamp, Gruyter & Verkley Synonym(s): Phoma exigua Desm. |
Yes (Parveen et al. 2019) | Yes. NSW, Qld, WA, Vic., Tas. (APPD 2022; Tran et al. 2013) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Helotiales: Sclerotiniaceae] Grey mould-rot |
Yes (Saranraj, Sivasakthivelan & Sivasakti 2016) | Yes. NSW, SA, Vic., Tas., WA (APPD 2022; Government of Western Australia 2022; Lindbeck, Bretag & Ford 2009) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Khare et al. 2016) | Yes. NSW, Qld (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Synonym(s): Chaetomium affine Corda; Chaetomium olivaceum Cooke & Ellis [Sordariales: Chaetomiaceae] |
Yes (Kumar 2019) | Yes. NSW, Qld, WA, Vic. (APPD 2022; Rahmadi & Fleet 2008) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Wet rot; Choanephora pod rot |
Yes (Kumar 2019) | Yes. NSW, Qld (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Choanephora infundibulifera (Curr.) Sacc. |
Yes (Das et al. 2017; Farr & Rossman 2022) | Yes. Qld (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Cladosporium cladosporioides f. pisicola (W.C. Snyder); Cladosporium pisicola W.C. Snyder; Monilia humicola Oudem; Penicillium cladosporioides Fresen) [Capnodiales: Cladosporiaceae] |
Yes (Kumar et al. 2013b) | Yes. NSW, Qld, NT, WA, SA, Vic., Tas. (APPD 2022; Government of Western Australia 2022; Ma, de Silva & Taylor 2020) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Antagonist of Botrytis cinerea |
Yes (Pande 2008; Plantwise 2023) | Yes. Qld, WA, Vic., Tas. (APPD 2022; Maxwell & Scott 2008) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Curvularia lunata (Wakker) Boedijn |
Yes (Kumkum, Sindhu & Shagufta 1989) | Yes. NSW, Qld, NT, WA, Vic. (APPD 2022; Government of Western Australia 2022; Pak et al. 2017) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Sphaeria dematium Pers.; Exosporium dematium (Pers.) Link; Vermicularia dematium (Pers.) Fr.; Lasiella dematium (Pers.) Quél. [Glomerellales: Glomerellaceae] |
Yes (Khare et al. 2016) | Yes (NSW, Qld, NT, SA, Vic., Tas. (APPD 2022; Shivas et al. 2016; Washington et al. 2006) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Gautam 2014) | Yes. NSW, Qld, NT, WA, SA, Vic., Tas., (APPD 2022; Giblin, Coates & Irwin 2010; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Corynespora cassiicola (Berk. & M.A. Curtis) C.T. Wei Synonym(s): Cercospora melonis Cooke |
Yes (Kamei et al. 2019) | Yes. NSW, Qld, NT, Vic., WA (APPD 2022; Government of Western Australia 2022; Silva et al. 1995) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Pleosporales: Pleosporaceae] |
Yes (Busi et al. 2009) | Yes. Qld, Tas. (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Dendryphiella vinosa (Berk & M. A. Curtis) Reisinger & Ravenel Synonym(s): |
Yes (HerbIMI 2020; Nonzom & Sumbali 2014) | Yes. Qld (APPD 2022; Queensland Department of Agriculture 1995) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Erysiphales: Erysiphaceae] |
Yes (Hosagoudar 1991) | No records found | No. Fibroidium abelmoschi was reported to be present on leaves, stems and petioles, causing powdery mildew in okra plants (Hosagoudar 1991; Kumar 2019). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Fusarium chlamydosporum Wollenw. & Reinking, |
Yes (Khare et al. 2016) | Yes. NSW, Qld, SA, Vic., WA (APPD 2022; Burgess & Summerell 1992; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Selenosporium equiseti Corda; Fusarium bullatum Sherb; Fusarium equiseti var. bullatum (Wollenw.) Wollenw.; Fusarium equiseti var. bullatum (Sherb.) Wollenw.; Fusarium falcatum Appel & Wollenw.; Gibberella intricans Wollenw.; Fusoma pallidum Bonord [Hypocreales: Nectriaceae] |
Yes (Singha et al. 2016) | Yes. NSW, Qld, WA, SA, Vic., Tas. (APPD 2022; Burgess & Summerell 1992) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Bakanae disease of rice |
Yes (Jamadar, Ashok & Shamarao 2001; Prasad et al. 2000b) | Yes. NSW, WA (Government of Western Australia 2022; Liew et al. 2016) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Fusarium oxysporum f.sp. vasinfectum (G.F. Atk.) W.C. Snyder & H.N. Hansen |
Yes (Khare et al. 2016) | Yes. NSW, Qld (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Fusarium angustum Sherb [Hypocreales: Nectriaceae] |
Yes (Khare et al. 2016) | Yes. NSW, Qld, NT, WA, SA, Vic., Tas. (APPD 2022; Burgess & Summerell 1992) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Basal canker on hop |
Yes (Sagar et al. 2011) | Yes. NSW, WA, SA, Vic., Tas. (APPD 2022; Tan et al. 2011) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Geotrichum candidum Link |
Yes (Prakash et al. 2012) | Yes. NSW, Qld, WA, Vic., Tas. (APPD 2022; Government of Western Australia 2022; Shivas 1989) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Erysiphe cichoracearum DC; Golovinomyces ambrosiae (Schwein.) U. Braun & R.T.A. Cook; Oidium asteris punicei Peck [Erysiphales: Erysiphaceae] |
Yes (Khare et al. 2016) | Yes. NSW, Qld, WA, Vic., Tas. (APPD 2022; Cunnington, Lawrie & Pascoe 2010) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Powdery mildew |
Yes (HerbIMI 2020; Sujata et al. 2018) | Yes. Qld, SA, Vic. (APPD 2022; Cunnington, Lawrie & Pascoe 2005) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Lasiodiplodia theobromae (Pat.) Griffon & Maubl. |
Yes (Dayal & Srivastava 1973) | Yes. NSW, Qld, WA (APPD 2022), NT, SA (CABI 2022; Government of Western Australia 2022; Peterson et al. 1991) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Sphaerulina trifolii Rostr; Pseudoplea trifolii (Rostr.) Petr. [Pleosporales: Pleosporaceae] |
Yes (Potkar & Jadhav 2015) | Yes. NSW, Qld, WA, SA, Vic., Tas. (APPD 2022; Barbetti 2007; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Powdery mildew of cotton |
Yes (Ullasa et al. 1981) | Yes. NSW, Qld, NT, WA, Vic. (APPD 2022; Liberato 2006) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Macrophomina phaseolina (Tassi) Goid. |
Yes (Begum, Lokesh & Kumar 2005) | Yes. NSW, Qld, NT, WA, SA, Vic. (APPD 2022; Government of Western Australia 2022; Hutton, Gomez & Mattner 2013) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Synonym(s): Fusarium solani (Mart.) Sacc; Fusarium aduncisporum Weimer & Harter; Nectria bogoriensis Bernard; Nectria calonectricola Henn. [Hypocreales: Nectriaceae] |
Yes (Kapadiya et al. 2013) | Yes. NSW, NT, Qld, WA, SA, Vic., Tas. (Elmer et al. 1997; Government of Western Australia 2022; Liew et al. 2016; Sangalang et al. 1995) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Singh & Narain 2008) | Yes. NSW, Qld, NT, SA, Vic. (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Penicillium chrysogenum Thom Synonym(s): Penicillium brunneorubrum Dierckx; Penicillium chlorophaeum Biourge |
Yes (Kumar et al. 2013b) | Yes. NSW, Qld, Vic., Tas., WA (APPD 2022; Government of Western Australia 2022; Visagie et al. 2014) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Post-harvest decay |
Yes (Kumar 2019) | Yes. NSW, Qld, Vic. (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Penicillium digitatum (Pers.) Sacc. |
Yes (Sharma, Maharshi & Gaur 2012) | Yes. NSW, Qld, WA, SA, Vic. (APPD 2022; Cook & Dubé 1989; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Botryosphaeriales: Phyllostictaceae] Phyllosticta leaf spot |
Yes (Khare et al. 2016) | No records found | No. Phyllosticta hibiscina has been reported to cause leaf spot disease on okra (Khare et al. 2016; Texas A&M AgriLife Extension 2020). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Khare et al. 2016) | Yes. NSW, Qld, NT, WA, SA, Vic. (APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Podosphaera xanthii (Castagne) U. Braun & Shishkoff Synonym(s): Sphaerotheca caricae-papayae Tanda & U. Braun; Meliola calendulae Malbr. & Roum. |
Yes (Nayak & Bandamaravuri 2018) | Yes. Qld, NT, WA, Vic. (APPD 2022; Liberato, Shivas & Cunnington 2006) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Capnodiales: Mycosphaerellaceae] Leaf spot of okra |
Yes (Ganesha & Jayalakshmi 2017) | Yes. WA (APPD 2022) | No. Pseudocercospora abelmoschi is reported to affect leaves only (Ganesha & Jayalakshmi 2017; Kumar 2019). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Khare et al. 2016) | No records found | No. The spots caused by P. hibiscina produces dark olivaceous patches of mouldy growth on lower surface of the leaf. (Khare et al. 2016). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Pseudothielavia terricola (J.C. Gilman & E.V. Abbott) X. Wei Wang & Houbraken Synonym(s): Thielavia terricola (Gilman & |
Yes (Dayal & Srivastava 1973) | Yes. ACT, Tas. (APPD 2022; Shivas 1989) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Pythiales: Pythiaceae] Damping-off |
Yes (Ashwathi et al. 2017) | Yes. NSW, Qld, Vic., WA (APPD 2022; Cook & Dubé 1989; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Anitha & Tripathi 2000) | Yes. NSW, Qld, NT, WA, SA, Vic., Tas. (APPD 2022; Cook & Dubé 1989; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Rhizopus arrhizus A. Fisch. Synonym(s): Mucor arrhizus (A. Fisch.) Hagem; Rhizopus oryzae Went & Prins. Geerl.; Rhizopus tritici Saito |
Yes (Kumari, Jayachandran & Ghosh 2019) | Yes. NSW, Qld, Vic., WA (APPD 2022; DAFWA 2015; Kennedy et al. 2016) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Mucorales: Mucoraceae] Bulb rot |
Yes (Shukla et al. 2006) | Yes. NSW, Qld, NT, WA, Vic. (APPD 2022; Cook & Dubé 1989; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Bag & Dutta 2009) | Yes. NSW, Qld, WA, SA, Vic., Tas. (APPD 2022; Cook & Dubé 1989; Government of Western Australia 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Uromyces heterogeneus Cooke Synonym(s): Caeomurus heterogeneus (Cooke); Coeomurus heterogeneus (Cooke) Kuntze |
Yes (Khare et al. 2016) | No records found | No. Uromyces heterogeneus is reported to only affect the leaves of okra (Smart Gardener 2019). | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
[Glomerellales: Plectosphaerellaceae] Verticillium wilt |
Yes (Kumar, Tapwal & Borah 2012) | Yes. ACT, NSW, Qld, NT, SA, Vic., Tas. (APPD 2022; Shivas 1989) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| PHYTOPLASMAS | ||||||||||||||||||
| Yes (Kumar, Singh & Lakhanpaul 2012) |
No records found | Yes. It infects okra and symptoms include shortening of internodes, aggregation of leaves at the apical region, reduced leaf lamina, stem reddening, fruit bending, phyllody and stunting of plants (Kumar, Singh & Lakhanpaul 2012). Affected fruit show a distinct bend or extreme curling and are devoid of seeds, being replaced by thin placental extensions (Kumar, Singh & Lakhanpaul 2012). As this phytoplasma infects systemically, infected fruit could be exported. | No. Phytoplasmas are transmitted by phloem-feeding
insects (Marcone 2014). 16SrI (B) group
phytoplasmas are transmitted by a range of leafhoppers, primarily
Macrosteles fascifrons (Lee,
Gundersen-Rindal & Bertaccini 1998; Lee et
al. 2004a). The end use (consumption), short shelf life of okra
fruit (7–10 days), and the mode of transmission of this phytoplasma by
leafhopper vectors make this pest extremely unlikely to be able to
transfer to a suitable host in Australia. Vectors of this phytoplasma that may be present in Australia would be unlikely to feed on discarded, dehydrated okra fruit, as it is highly perishable and susceptible to water loss (Tamura & Minamide 1984). Huberty and Denno (2004) demonstrated vascular feeding arthropods experience negative responses when forced to feed on water-stressed hosts. |
Assessment not required | Assessment not required | No | ||||||||||||
| VIRUSES | ||||||||||||||||||
[Bromoviridae: Cucumovirus] |
Yes (Kumar, Gautam & Raj 2014; Lepcha, Chaudhary & Pratap 2017) | Yes. NSW, Qld, SA, Tas., Vic., WA (Alberts, Hannay & Randles 1985; APPD 2022) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Okra yellow vein mosaic virus (OYVMV) |
Yes (Ansar et al. 2014; Solankey, Singh & Singh 2016) | No records found | Yes. OYVMV disease causes homogenous yellowing of veins in leaf tissue that become yellowish/creamy colour, which later become necrotic. It causes stunting okra (Ali et al. 2012; Plantwise 2023; Venkataravanappa et al. 2015). It is unlikely that these viruses will be present on the pathway, as fruit from infected plants are yellow or white in colour making them unmarketable (Venkataravanappa et al. 2015). However, fruit at the early stage of the infection may show no obvious symptoms; therefore, may not be removed during harvest and post-harvest processes and potentially be exported. | No. Yellow vein mosaic virus disease of okra spreads in
areas with high rainfall and humidity and is transmitted by whitefly,
Bemisia tabaci (Gilbertson et al. 2015). OYVMV
is not known to be seed transmitted and imported okra fruit have
immature seeds that are not able to germinate (Bortey & Dzomeku 2016; Demir 1994). The end use, short shelf life of
okra fruit (7–10 days), and the mode of transmission of OYVMV by
grafting and whitefly vectors make this pest extremely unlikely to be
able to transfer to a suitable host in Australia (Gilbertson et al. 2015). Vectors of this virus that may be present in Australia would be unlikely to feed on discarded, dehydrated okra fruit, as it is highly perishable and susceptible to water loss (Tamura & Minamide 1984). Huberty and Denno (2004) demonstrated vascular feeding arthropods experience negative responses when forced to feed on water-stressed hosts. |
Assessment not required | Assessment not required | No | |||||||||||
| Yes (Kumar et al. 2012) | No records found | No. Characteristic symptoms of this disease on okra include leaf curling and overall stunting of plants that bear no fruit (Kumar et al. 2012). | Assessment not required | Assessment not required | Assessment not required | No | ||||||||||||
Tobacco streak virus (TSV) |
Yes (Krishnareddy, Jalali & Samuel 2007; Vemana & Jain 2010) | Yes. Qld (Sharman, Thomas & Persley 2008) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
| Yes (Venkataravanappa et al. 2012b) | No records found | As this virus infects plants systemically, in theory, there is a possibility of the virus being present in fruit. Fruit harvested from infected plants especially at the early stage of the infection may show no obvious symptoms; therefore, may not be removed during harvest and post-harvest processes and potentially be exported. |
Assessment not required | Assessment not required | No | |||||||||||||
[Potyviridae; Potyvirus] |
Yes (Mongamaithem & Rebika 2018; Singh et al. 2015) | Yes. NSW, SA, Vic., WA (Coutts, Walsh & Jones 2007; Government of Western Australia 2022; Persley, Cooke & House 2010; Schwinghamer et al. 2014) | Assessment not required | Assessment not required | Assessment not required | Assessment not required | No | |||||||||||
Okra Enation Leaf Curl Virus |
Yes (Chandran et al. 2013) | No records found | No. OELCuV is not known to be seed transmitted and
imported okra fruit have immature seeds that are not able to germinate
(Bortey & Dzomeku 2016; Demir 1994). The end use, short shelf life of
okra fruit (7–10 days), and the mode of transmission of OELCuV by
grafting and whitefly vectors make this pest extremely unlikely to be
able to transfer to a suitable host in Australia (Gilbertson et al. 2015). Vectors of this virus that may be present in Australia would be unlikely to feed on discarded, dehydrated okra fruit, as it is highly perishable and susceptible to water loss (Tamura & Minamide 1984). Huberty and Denno (2004) demonstrated vascular feeding arthropods experience negative responses when forced to feed on water-stressed hosts. |
Assessment not required | Assessment not required | No | ||||||||||||
Bhendi yellow vein mosaic Delhi virus [BYVDV-IN (India: Delhi: okra)] |
Yes (Venkataravanappa et al. 2012b) | No records found | Huberty and Denno (2004) demonstrated vascular feeding arthropods experience negative responses when forced to feed on water-stressed hosts. |
Assessment not required | Assessment not required | No | ||||||||||||
Bhendi yellow vein Bhubhaneswar virus |
Yes (Venkataravanappa et al. 2013) | No records found | No. BYVBV is not known to be seed transmitted and
imported okra fruit have immature seeds that are not able to germinate
(Bortey & Dzomeku 2016; Demir 1994). The end use, short shelf life of
okra fruit (7–10 days), and the mode of transmission of BYVBV by
grafting and whitefly vectors make this pest extremely unlikely to be
able to transfer to a suitable host in Australia (Gilbertson et al. 2015). Vectors of this virus that may be present in Australia would be unlikely to feed on discarded, dehydrated okra fruit, as it is highly perishable and susceptible to water loss (Tamura & Minamide 1984). Huberty and Denno (2004) demonstrated vascular feeding arthropods experience negative responses when forced to feed on water-stressed hosts. |
Assessment not required | Assessment not required | No | ||||||||||||
Bhendi yellow vein Madurai virus (BYVMV) |
Yes (Venkataravanappa et al. 2015) | No records found | No. BYVMV is not known to be seed transmitted and
imported okra fruit have immature seeds that are not able to germinate
(Bortey & Dzomeku 2016; Demir 1994). The end use, short shelf life of
okra fruit (7–10 days), and the mode of transmission of BYVMV by
grafting and whitefly vectors make this pest extremely unlikely to be
able to transfer to a suitable host in Australia (Gilbertson et al. 2015). Vectors of this virus that may be present in Australia would be unlikely to feed on discarded, dehydrated okra fruit, as it is highly perishable and susceptible to water loss (Tamura & Minamide 1984). Huberty and Denno (2004) demonstrated vascular feeding arthropods experience negative responses when forced to feed on water-stressed hosts. |
Assessment not required | Assessment not required | No | ||||||||||||
(BYVMaV) [Geminiviridae: Begomovirus] |
Yes (Venkataravanappa et al. 2015) | No records found | No. BYVMaV is not known to be seed transmitted and
imported okra fruit have immature seeds that are not able to germinate
(Bortey & Dzomeku 2016; Demir 1994). The end use, short shelf life of
okra fruit (7–10 days), and the mode of transmission of BYVMaV by
grafting and whitefly vectors make this pest extremely unlikely to be
able to transfer to a suitable host in Australia (Gilbertson et al. 2015). Vectors of this virus that may be present in Australia would be unlikely to feed on discarded, dehydrated okra fruit, as it is highly perishable and susceptible to water loss (Tamura & Minamide 1984). Huberty and Denno (2004) demonstrated vascular feeding arthropods experience negative responses when forced to feed on water-stressed hosts. |
Assessment not required | Assessment not required | No | ||||||||||||
[Geminiviridae: Begomovirus] |
Yes (Venkataravanappa et al. 2012a) | No records found | Yes. CLCuAV infected okra plants exhibit mottling, downward leaf curling, vein thickening and twisting and yellowing symptoms (Venkataravanappa et al. 2012a). It is unlikely that CLCuAV will be present on the pathway, as virus infected fruit are largely deformed and unmarketable and likely to be removed following packing house quality grading practices. |
Assessment not required | Assessment not required | No | ||||||||||||
Bhendi yellow vein Haryana virus [BYVHV (India: Haryana:06)] |
Yes (Venkataravanappa et al. 2015) | No records found | No. BYVHV is not known to be seed transmitted and
imported okra fruit have immature seeds that are not able to germinate
(Bortey & Dzomeku 2016; Demir 1994). The end use, short shelf life of
okra fruit (7–10 days), and the mode of transmission of BYVHV by
grafting and whitefly vectors make this pest extremely unlikely to be
able to transfer to a suitable host in Australia (Gilbertson et al. 2015). Vectors of this virus that may be present in Australia would be unlikely to feed on discarded, dehydrated okra fruit, as it is highly perishable and susceptible to water loss (Tamura & Minamide 1984). Huberty and Denno (2004) demonstrated vascular feeding arthropods experience negative responses when forced to feed on water-stressed hosts. |
Assessment not required | Assessment not required | No | ||||||||||||
(BYVKnV) [Geminiviridae: Begomovirus] |
Yes (Venkataravanappa et al. 2015) | No records found | No. BYVKnV is not known to be seed transmitted and
imported okra fruit have immature seeds that are not able to germinate
(Bortey & Dzomeku 2016; Demir 1994). The end use, short shelf life of
okra fruit (7–10 days), and the mode of transmission of BYVKnV by
grafting and whitefly vectors make this pest extremely unlikely to be
able to transfer to a suitable host in Australia (Gilbertson et al. 2015). Vectors of this virus that may be present in Australia would be unlikely to feed on discarded, dehydrated okra fruit, as it is highly perishable and susceptible to water loss (Tamura & Minamide 1984). Huberty and Denno (2004) demonstrated vascular feeding arthropods experience negative responses when forced to feed on water-stressed hosts. |
Assessment not required | Assessment not required | No | ||||||||||||
Appendix C: Stakeholder comments
The department received a comment on the published draft report, suggesting that the above two spider mites are unlikely to be associated with okra fruit, as they are primarily foliage pests and are likely to be removed during packing house processes.
The department reviewed the evidence for the presence of spider mites in India and their association with okra fruit. Further examination of the literature supports the original assessment and the association of these pests with okra fruit; therefore, the originally assessed risk ratings have been retained.
Glossary, acronyms and abbreviations
References
All web links in references were accessible and active on week of 15 January 2023.
Alberts, E, Hannay, J & Randles, JW 1985, ‘An epidemic of cucumber mosaic virus in South Australian lupins’, Australian Journal of Agricultural Research, vol. 36, no. 2, pp. 267-73. (Abstract only)
Alcock, J 1971, ‘The behavior of a stinkbug, Euschistus conspersus Uhler (Hemiptera: Pentatomidae)’, Psyche, vol. 78, no. 4, pp. 215-28.
Ananda, N 2007, ‘Seasonal incidence and management of sucking pests of pomegranate’, Master of Science (Agriculture) Thesis, University of Agricultural Sciences, Dharwad.
Anderson, NL 1964, ‘Observations on some grasshoppers of the Rukwa Valley, Tanganyika’, Proceedings of the Zoological Society of London, vol. 143, no. 3, pp. 395-403.
-- -- 2021, ‘Product profile: Indian okra’, Agricultural and Processed Food Products Export Development Authority Agri-exchange, India, available at https://agriexchange.apeda.gov.in/India%20Production/India_Productions.aspx?cat=Vegetables&hscode=1079.
APPD 2022, ‘Australian Plant Pest Database, online database’, available at https://www.appd.net.au/, accessed 2022.
Babu, GP, Chakravarthy, D, Kumar, KJ & Paramageetham, C 2013, ‘Biochemical characterization of phosphate degrading Pseudomonas cichorii isolated from forest soils in Seshachalam Hills’, Research and Reviews: Journal of Microbiology and Biotechnology, vol. 2, no. 1, pp. 27-30.
Bachhar, A, Bhaskar, H, Pathrose, B & Shylaja, MR 2019, ‘Status of Acaricide resistance in Tetranychus truncatus Ehara (Prostigmata: Tetranychidae) on vegetable crops on Thrissur district, Kerala’, Indian Journal of Entomology, vol. 81, no. 1, pp. 130-3.
Banjo, AD 2010, ‘A review of on Aleurodicus dispersus Russel. (spiralling whitefly) [Hemiptera: Aleyrodidae] in Nigeria’, Journal of Entomology and Nematology, vol. 2, no. 1, pp. 001-6.
Barber, PA, Paap, T, Burgess, TI, Dunstan, W & Hardy, GE St. J 2013, ‘A diverse range of Phytophthora species are associated with dying urban trees’, Urban Forestry & Urban Greening, vol. 12, pp. 569-75.
Bellis, GA, Donaldson, JF, Carver, M, Hancock, DL & Fletcher, MJ 2004, ‘Records of insect pests on Christmas Island and the Cocos (Keeling) Islands, Indian Ocean’, The Australian Entomologist, vol. 31, pp. 93-102.
Ben-Dov, Y 1993, A systematic catalogue of the soft scale insects of the world (Homoptera: Coccoidea: Coccidae) with data on geographical distribution, host plants, biology and economic importance, vol. 9, Arnett, RH, Jr (ed), Sandhill Crane Press, Inc., Gainesville.
-- -- 2011, Revised conditions for importing fresh mango fruit from India, final report, Department of Agriculture, Fisheries and Forestry, Canberra, available at https://www.agriculture.gov.au/biosecurity-trade/policy/risk-analysis/plant/mangoes-from-india.
Biosecurity Tasmania 2021, Fall armyworm, Department of Primary Industries, Parks, Water and Environment (Tasmania), Tasmania, Australia, https://nre.tas.gov.au/biosecurity-tasmania/plant-biosecurity/pests-and-diseases#Fallarmyworm.
Borkar, AN, Kolhe, AV & Undirwade, DB 2020, ‘Biology and life studies of Tetranychus macfarlanei on okra’, Journal of Entomology and Zoological Studies, vol. 8, no. 5, pp. 2411-5.
Borowiec, L 1985, ‘On the Oriental Spermophagus Schoenherr (Coleoptera, Bruchidae, Amblycerinae), with description of four new species’, Polskie Pismo Entomologiczne, vol. 55, pp. 781-90.
Broadbent, P, Baker, KF & Waterworth, Y 1971, ‘Bacteria and actinomycetes antagonistic to fungal root pathogens in Australian soils’, Australian Journal of Biological Sciences, vol. 24, pp. 925-44.
Brunner, JF 1993, Leafrollers, Orchard Pest Management Online, Washington State University, available at http://treefruit.wsu.edu/crop-protection/opm/leafrollers/?print-view=true.
Butani, DK & Jotwani, MG 1984, ‘Okra’, in Insects in vegetables, Periodical Expert Book Agency, New Delhi.
CABI-EPPO 1997, ‘Aulacophora indica [Distribution map]’, CABI-EPPO, Wallingford; UKavailable at https://www.gbif.org/species/5876578.
Chakraborty, A, Kumar, K & Rajadurai, G 2014, ‘Biodiversity of insect fauna in okra (Abelmoschus esculentus (L.) Moench) ecosystem’, Trends in Biosciences, vol. 7, no. 16, pp. 2206-11.
Chakravarthy, AK & Sridhara, S 2016, Economic and ecological significance of arthropods in diversified ecosystems - sustaining regulatory mechanisms, Chakravarthy, AK & Sridhara, S (eds), Springer, Singapore.
Chittora, A & Singh, N 2016, ‘Production technology of okra’, Marumegh, vol. 1, no. 1, pp. 48-51.
Chowda-Reddy, RV, Kirankumar, M, Seal, SE, Muniyappa, V, Valand, GB, Govindappa, MR & Colvin, J 2012, ‘Bemisia tabaci phylogenetic groups in India and the relative transmission efficacy of Tomato leaf curl Bangalore virus by an indigenous and an exotic population’, Journal of Integrative Agriculture, vol. 11, no. 2, pp. 235-48.
Climate-data.org 2021, ‘Climate-data.org - climate data for cities worldwide’, AM Online Projects, available at https://en.climate-data.org/, accessed 2021.
Colt, M, Fallahi, E, Himyck, R & Lyon, T 2001, Idaho crop profiles: apples, College of Agricultural and Life Sciences, University of Idaho, USA, CIS 1090.
Cranham, JE & Danthanarayana, W 1971, ‘Tea tortrix (Homona coffearia Nietner)’, Advisory Pamphlet, Tea Research Institute of Ceylon, vol. 5, no. 66, p. 8. (Abstract only)
Cunnington, JH, Lawrie, AC & Pascoe, IG 2005, ‘Molecular identification of Golovinomyces (Ascomycota: Erysiphales) anamorphs on the Solanaceae in Australia’, Australasian Plant Pathology, vol. 34, no. 1, pp. 51-5.
DAF 2013, Solenopsis mealybug in Australia – an overview, Queensland Government Department of Agriculture and Fisheries, Queensland, Australia.
DAFF 2003, Pest and Disease Information Database: pest interception records up to 31 December, 2002, Department of Agriculture, Fisheries and Forestry.
Daravath, V, Kasbe, SS & Musapuri, S 2020, ‘Flower chafer beetle (Oxycetonia versicolor Fabricius) on the verge of becoming a major pest on cotton in Telangana region of India: a first report’, Journal of Entomology and Zoological Studies, vol. 8, no. 2, pp. 242-6.
Das, S, Dutta, S, Chattopadhyay, A & Mandal, B 2017, ‘First report of Choanephora infundibulifera causing blossom blight of teasle gourd in India’, Indian Phytopathology, vol. 70, no. 2, pp. 265-7.
DAWR 2015, Final report for the non-regulated analysis of existing policy for fresh mango fruit from Indonesia, Thailand and Vietnam, Australian Government Department of Agriculture and Water Resources, Canberra, available at https://www.agriculture.gov.au/biosecurity-trade/policy/risk-analysis/plant/mangosteens-indonesia (pdf 3.6 mb).
-- -- 2016, Final report for the non-regulated analysis of existing policy for table grapes from India, the Australian Government Department of Agriculture and Water Resources, Canberra, available at https://www.agriculture.gov.au/biosecurity-trade/policy/risk-analysis/memos/ba2016-25.
De Meyer, M, Delatte, H, Mwatawala, M, Quilici, S, Vayssieres, JF & Virgilio, M 2015, ‘A review of the current knowledge on Zeugodacus cucurbitae (Coquillett) (Diptera, Tephritidae) in Africa, with a list of species included in Zeugodacus’, ZooKeys, vol. 540, pp. 539-57.
De Prins, J & De Prins, W 2022, ‘Afromoths: online database of Afrotropical moth species (Lepidoptera)’, Belgian Biodiversity Platform, Brussels, Belgium, available at https://www.afromoths.net/, accessed 2022.
Dhankhar, BS & Mishra, JP 2005, ‘Objectives of okra breeding’, Journal of New Seeds, vol. 6, no. 2-3, pp. 195-209.
Dhawan, AK & Sidhu, AS 1984, ‘Incidence and relative abundance of different species of spotted bollworms on okra at Ludhiana, Punjab’, Journal of Research, Punjab Agricultural University, vol. 21, no. 4, pp. 533-42. (Abstract only)
DPIRD 2021, ‘American serpentine leafminer confirmed in Western Australia’, Department of Primary Industries and Regional Development, Western Australia, available at https://www.wa.gov.au/government/announcements/american-serpentine-leafminer-confirmed-western-australia.
DPP 2007, Export of grapes from India to Australia, Directorate of Plant Protection, Ministry of Agriculture, India.
El-Gendy, IR 2017, ‘Host preference of the peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae), under laboratory conditions’, Journal of Entomology, vol. 14, pp. 160-7.
Elhalis, H, Cox, J & Zhao, J 2020, ‘Ecological diversity, evolution and metabolism of microbial communities inthe wet fermentation of Australian coffee beans’, International Journal of Food Microbiology, vol. 321.
-- -- 2021, ‘EPPO Global Database’, European and Mediterranean Plant Protection Organization (EPPO), available at https://gd.eppo.int/, accessed 2021.
FAO 2016a, International Standards for Phytosanitary Measures (ISPM) no. 10: Requirements for the establishment of pest free places of production and pest free production sites, Secretariat of the International Plant Protection Convention, Food and Agriculture Organization of the United Nations, Rome, Italy, available at https://www.ippc.int/en/core-activities/standards-setting/ispms/.
-- -- 2019a, International Standards for Phytosanitary Measures (ISPM) no. 2: Framework for pest risk analysis, Secretariat of the International Plant Protection Convention, Food and Agriculture Organization of the United Nations, Rome, Italy, available at https://www.ippc.int/en/core-activities/standards-setting/ispms/.
-- -- 2019b, International Standards for Phytosanitary Measures (ISPM) no. 11: Pest risk analysis for quarantine pests, Secretariat of the International Plant Protection Convention, Food and Agriculture Organization (FAO) of the United Nations, Rome, Italy, available at https://www.ippc.int/en/core-activities/standards-setting/ispms/.
-- -- 2022, ‘Fungal Databases’, U.S. National Fungal Collections, ARS, USDA, available at https://nt.ars-grin.gov/fungaldatabases/, accessed 2022.
FDACS 2017, ‘Fruit Fly Pests’, Florida Department of Agriculture and Consumer Services, USA, available at https://www.fdacs.gov/content/download/9756/file/FruitFlyPests.pdf (pdf 2.2 mb).
Fluidquip Australia 2009, ‘Common water-borne bacteria’, available at https://www.fluidquip.com.au/food-beverage-pharma/medical-opthalmic/common-water-borne-micro-organisms/common-water-borne-bacteria.
Follett, PA, Jamieson, L, Hamilton, L & Wall, M 2019, ‘New associations and host status: infestability of kiwifruit by the fruit fly species Bactrocera dorsalis, Zeugodacus cucurbitae, and Ceratitis capitata (Diptera: Tephritidae)’, Crop Protection, vol. 115, no. 1, pp. 113-21.
Gautam, AK 2014, ‘Colletotrichum gloeosporioides: biology, pathogenicity and management in India’, Journal of Plant Physiology and Pathology, vol. 2, no. 2.
Geiser, DM, Pitt, JI & Taylor, JW 1998, ‘Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus’, Proceedings of the National Academy of Sciences, vol. 95, no. 1, pp. 388-93.
Gilbertson, RL, Batuman, O, Webster, CG & Adkins, S 2015, ‘Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses’, The Annual Review of Virology, vol. 2, no. 1, pp. 67-93.
Gilligan, TM, Baixeras, J & Brown, JW 2018, ‘T@RTS: Online World Catalogue of the Tortricidae (ver. 4.0)’, available at http://www.tortricidae.com/catalogue.asp, accessed 2020.
-- -- 2017a, Technical information on okra (Abelmoschus esculentum L.) for export to Australia, Ministry of Agriculture and Farmers Welfare, New Delhi, India.
-- -- 2017b, Technical information on pomegranate fruits and arils (processed ready-to-eat) for export to Australia, Ministry of Agriculture & Farmers Welfare, India.
Gullan, PJ, Miller, DR & Cook, LG 2005, ‘Gall-inducing scale insects (Hemiptera: Sternorryncha: Coccoidea)’, in Biology, ecology, and evolution of gallinducing arthropods, Raman, A, Schaefer, CW & Withers, TM (eds), Science Publishers, Inc., Plymouth, UK.
Gupta, R, Tara, JS & Pathania, PC 2013, ‘First report of yellow tail tussock moth, Somena scintillans Walker (Lepidoptera: Lymantriidae) on apple plantations in Jammu’, Journal of Insect Science, vol. 28, no. 1, pp. 130-3.
Gupta, SL 1990, ‘Key for the identity of some major lepidopterous pests of vegetables in India’, Bulletin of Entomology, vol. 31, no. 1, pp. 69-84.
Gurney, WB 1924, ‘Insect Pests of Cotton in New South Wales’, Agricultural Gazette of New South Wales, vol. 35, no. 2, pp. 137-8. (Abstract only)
Harteveld, DOC, Akinsanmi, OA & Drenth, A 2013, ‘Multiple Alternaria species groups are associated with leaf blotch and fruit spot diseases of apple in Australia’, Plant pathology, vol. 62, pp. 289-97.
Harvey, JA, Nehl, DB & Aitken, EA 2004, ‘Occurrence of the black root rot: a pandemic in Australian cotton’, Australasian Plant Pathology, vol. 33, no. 1, pp. 87-95.
Herbison-Evans, D & Crossley, S 2022, ‘Australian Caterpillars and their Butterflies and Moths’, Coffs Harbour Butterfly House, Coffs Harbour NSW, Australia, available at http://lepidoptera.butterflyhouse.com.au/, accessed 2022.
Hey, J 2015, ‘India suspends okra exports’, Fresh Produce Journal, Britain, available at http://www.fruitnet.com/fpj/article/164816/india-suspends-okra-exports.
Hosagoudar, VB 1991, ‘Some powdery mildews from Tamil Nadu, India’, Sydowia, vol. 43, pp. 23-30.
Huberty, AF & Denno, RF 2004, ‘Plant water stress and its consequences for herbivorous insects: a new synthesis’, Ecology, vol. 85, no. 5, pp. 1383-98.
Infonet 2019, Okra, Infonet-Biovision, Switzerland, https://infonet-biovision.org/PlantHealth/Crops/Okra.
IPPC 2020, Further detections of Spodoptera frugiperda (fallarmyworm) on mainland Australia, AUS-98/1, International Plant Protection Convention, Rome, Italy, available at https://www.ippc.int/en/countries/Australia/pestreports/2020/05/further-detections-of-spodoptera-frugiperda-fall-armyworm-on-mainland-australia/.
Jamadar, MM, Ashok, S & Shamarao, J 2001, ‘Studies on seed mycoflora and nematodes and their effect on germination and vigour index of colour graded okra [Abelmoschus esculentus (L.) Moench]’, Crop Research (Hisar), vol. 22, no. 3, pp. 479-84.
James, DG, Bartelt, RJ, Faulder, RJ & Taylor, A 1993, ‘Attraction of Australian Carpophilus spp. (Coleoptera: Nitidulidae) to synthetic pheromones and fermenting bread dough’, Journal of Australian Entomological Society, vol. 32, pp. 339-45.
Job, M, Singh, VK & Dinmani 2018, ‘Study of water and nutrients requirement through drip irrigation in okra’, Journal of Pharmacognosy and Phytochemistry, vol. SP1, pp. 3172-6.
Joshi, KC & Khan, HR 1990, ‘Biology and control of the giant red bug Lohita grandis Gray (Hemiptera: Pyrrhocoridae: Largidae)’, Indian Forester, vol. 116, no. 4, pp. 312-9.
Kapadiya, IB, Akbari, LF, Siddhapara, MR & Undhad, SV 2013, ‘Evaluation of fungicides and herbicides against the root rot of okra’, The Bioscan, vol. 8, no. 2, pp. 433-6.
Kapoor, VC 2002, ‘Fruit-fly pests and their present status in India’, Proceedings of 6th International Fruit Fly Symposium, Stellenbosch, South Africa, 6-10 May 2002, pp. 23-33.
Kelkar, AP, Munj, AY, Desai, VS, Golvankar, GM & Shinde, PB 2018, ‘Management of okra flea beetle (Podagrica bowringi) Baly by using different insecticidal dust, botanical and entomopathogenic fungi’, International Journal of Chemical Studies, vol. 6, no. 6, pp. 2038-41.
Kennedy, GG & Smitley, DR 1985, ‘Dispersal’, in Spider mites: their biology, natural enemies and control. Vol. 1A, Helle, W & Sabelis, MW (eds), Elsevier Science Publishers B.V., Amsterdam.
Konar, A & Rai, L 1990, ‘Efficacy of some insecticides against shoot and fruit borer (Earias vittella Fab. and Earias insulana Boisd.) of okra (Abelmoschus esculentus L. Moench)’, Environment and Ecology, vol. 8, no. 1B, pp. 410-3. (Abstract only)
Konar, A & Roy, PS 2008, ‘Studies on the incidence of parasites of scale insects infesting orange in Darjeeling, West Bengal’, Journal of Entomological Research (New Delhi), vol. 32, no. 3, pp. 193-9.
Kuhar, TP, Kamminga, KL, Whalen, J, Dively, GP, Brust, G, Hooks, CRR, Hamilton, G & Herbert, DA 2012, ‘The pest potential of brown marmorated stink bug on vegetable crops’, Plant Health Progress, vol. May 2012, DOI 10.1094/PHP-2012-0523-01-BR.
Kulkarni, AU & Chavan, AM 2010, ‘Fungal load on Zea mays seeds and their biocontrol’, Journal of Experimental Sciences, vol. 1, no. 2, pp. 20-5.
Kumar, H & Usmani, MK 2014, ‘Taxonomic studies on Acrididae (Orthoptera: Acridoidea) from Rajasthan (India)’, Journal of Entomology and Zoology Studies, vol. 2, no. 3, pp. 131-46.
Kumar, J, Kumar, A, Singh, SP, Roy, JK, Lalit, A, Parmar, D, Sharma, NC & Tuli, R 2012, ‘First report of Radish leaf curl virus infecting okra in India’, New Disease Reports, vol. 25, 9, http://dx.doi.org/10.5197/j.2044-0588.2012.025.009.
Kumar, S, Gautam, KK & Raj, SK 2014, ‘Molecular identification of Cucumber mosaic virus isolates of subgroup IB associated with mosaic disease of eggplant in India’, VirusDisease, vol. 25, no. 1, pp. 129-31.
Kumar, S, Singh, V & Lakhanpaul, S 2012, ‘Molecular characterization and phylogeny of phytoplasma associated with bunchy top disease in its new host Okra (Abelmoschus esculentus) in India reveal a novel lineage within the 16SrI group’, European Journal of Plant Pathology, vol. 133, pp. 371-8.
Kumawat, KC & Singh, SP 2002, ‘Evaluation of insecticides and acaricides against oriental mite infesting pomegranate’, Annals of Plant Protection Sciences, vol. 10, no. 1, pp. 134-78.
Kumkum, G, Sindhu, IR & Shagufta, N 1989, ‘Seed mycoflora of Abelmoschus esculentus (L.) Moench: 1. survey and enumeration’, Acta Botanica Indica, vol. 17, no. 2, pp. 200-6. (Abstract only)
Lee, IM, Gundersen-Rindal, DE & Bertaccini, A 1998, ‘Phytoplasma: ecology and genomic diversity’, Phytopathology, vol. 88, no. 12, pp. 1359-66.
Lee, IM, Gundersen-Rindal, DE, Davis, RE, Bottner, KD, Marcone, C & Seemüller, E 2004a, ‘'Candidatus Phytoplasma asteris', a novel phytoplasma taxon associated with aster yellows and related diseases’, International Journal of Systematic and Evolutionary Microbiology, vol. 54, no. 4, pp. 1037-48.
Liberato, JR, Shivas, RG & Cunnington, JH 2006, ‘Podosphaera xanthii on Euryops chrysanthemoides in Australia’, Australasian Plant Pathology, vol. 35, no. 6, pp. 739-41.
Liew, ECY, Laurence, MH, Pearce, CA, Shivas, RG, Johnson, GI, Tan, YP, Edwards, J, Perry, S, Cooke, AW & Summerell, BA 2016, ‘Review of Fusarium species isolated in association with mango malformation in Australia’, Australasian Plant Pathology, vol. 45, https://link.springer.com/article/10.1007/s13313-016-0454-z.
Luna, RK, Ajay, S, Sehgal, RN, Rakesh, K & Rekesh, G 2008, ‘Bioefficacy of drek (Melia azedarach) seeds against red pumpkin beetel, Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae)’, Indian Journal of Forestry, vol. 31, no. 3, pp. 357-60. (Abstract only)
Ma, M, de Silva, DD & Taylor, PWJ 2020, ‘Black mould of post-harvest tomato (Solanum lycopersicum) caused by Cladosporium cladosporioides in Australia’, Australasian Plant Disease Notes, vol. 15, 25, https://doi.org/10.1007/s13314-020-00395-8.
Mainali, RP 2014, ‘Biology and management of eggplant fruit and shoot borer, Leucinodes orbonalis Guenee (Lepidoptera: Pyralidae): a review’, International Journal of Applied Science and Biotechnology, vol. 2, no. 1, pp. 18-28.
Maisuria, VB & Nerurkar, AS 2013, ‘Characterization and differentiation of soft rot causing Pectobacterium carotovorum of Indian origin’, European Journal of Plant Pathology, vol. 136, pp. 87-102.
Mani, M, Shivaraju, C & Shylesha, AN 2012, ‘Paracoccus marginatus, an invasive mealybug of papaya and its biological control - an overview’, Journal of Biological Control, vol. 26, no. 3, pp. 201-16.
Manjunatha, HA, Patil, SB, Udikeri, SS & Jahagirdhar, S 2017, ‘Study on bioefficacy of insecticides against shoot weevil (Alcidodes affaber Aurivillius) in okra (Abelmoschus esculentus Linn.)’, International Journal of Current Microbiology and Applied Sciences, vol. 6, no. 9, pp. 3447-56.
Martin Kessing, JL & Mau, RFL 2007, Trialeurodes vaporariorum (Westwood), Crop Knowledge Master.
Maxwell, A & Scott, JK 2008, ‘Pathogens on wild radish, Raphanus raphanistrum (Brassicaceae), in south-western Australia – implications for biological control’, Australasian Plant Pathology, vol. 37, pp. 523-33.
Miyazaki, M & Kudo, I 1988, ‘Bibliography and host plant catalogue of Thysanoptera of Japan’ (in Japanese), National Institute of Agro-Environmental Sciences, vol. 3, pp. 1-246.
Mkiga, AM & Mwatawala, MW 2015, ‘Developmental biology of Zeugodacus cucurbitae (Diptera: Tephritidae) in three cucurbitaceous hosts at different temperature regimes’, Journal of Insect Science, vol. 15, no. 1, DOI 10.1093/jisesa/iev141.
Moslemi, A, Ades, PK, Groom, T, Nicolas, ME & Taylor, PWJ 2017, ‘Alternaria infectoria and Stemphylium herbarum, two pathogens of pyrethrum (Tanacetum cinerariifolium) in Australia’, Australasian Plant Pathology, vol. 46, pp. 91-101.
Mound, LA, Tree, DJ & Paris, D 2018, ‘Oz Thrips: Thysanoptera in Australia’, available at http://www.ozthrips.org/, accessed 2018.
Munthali, DC & Tshegofatso, AB 2013, ‘Major insect pests attacking okra; Abelmoschus esculentus (L) Moench, in Sebele; Botswana’, Botswana Journal of Agricultural & Applied Sciences, vol. 9, no. 2, pp. 90-6.
Mursal, EI 2000, ‘Comparative studies on the biology and morphology of Earias insulana (Boisd.) and Earias vittella (Fab.) (Lepidoptera: Noctuidae) reared on okra’, Masters of Science (Crop Protection) Thesis, University of Khartoum.
Nair, N, Giri, U, Bhattacharjee, T, Thangjam, B, Paul, N & Debnath, MR 2017, ‘Biodiversity of insect pest complex infesting okra (Abelmoschus esculentus) in Tripura, N.E. India’, Journal of Entomology and Zoology Studies, vol. 5, no. 5, pp. 1968-72.
Nakahara, S 1982, Checklist of the armored scales (Homoptera: Diaspididae) of the conterminous United States, United States Department of Agriculture, Hoboken.
Neal, A 2017, ‘Featured Creatures - Sri Lankan weevil, Myllocerus undecimpustulatus undatus Marshall (Insecta: Coleoptera: Curculionidae: Entiminae)’, University of Florida, Florida, USA, available at http://entnemdept.ufl.edu/creatures/orn/sri_lankan_weevil.htm.
Netam, PK, Ganguli, RN & Dubey, AK 2007, ‘Insect pest succession in okra’, Environment & Ecology, vol. 25, no. 1, pp. 177-80.
Obeng-Ofori, D & Sackey, J 2003, ‘Field evaluation of non-synthetic insecticides for the management of insect pests of okra, Abelmoschus esculentus (L.) Moench in Ghana’, SINET: Ethiopian Journal of Science, vol. 26, no. 2, pp. 145-50.
PaDIL 2020, ‘PaDIL website’, Australian Governement Department of Agriculture, Water and the Environment, available at http://www.padil.gov.au, accessed 2020.
Pang, BP, X.R., Z, Shi, L & Mu, HB 2004, ‘Performance of Tetranychus truncatus Ehara (Acarina: Tetranychidae) reared with different host plants’ (in Chinese), Acta Entomologica Sinica, vol. 47, no. 1, pp. 55-8.
Pant, CP 1960, ‘Some aspects of the bionomics of Earias spp. at Kanpur’, Agra University Journal of Research (Science), vol. 9, no. 1, pp. 31-40.
Pathania, PC, Das, A, Brown, JW & Chandra, K 2020, ‘Catalogue of Tortricidae Latreille, 1802 (Lepidoptera: Tortricoidea) of India’, Zootaxa, vol. 4757, no. 1, pp. 001-95.
Persley, D, Cooke, T & House, S 2010, Diseases of vegetable crops in Australia, Commonwealth Scientific and Industrial Research Organisation, Collingwood.
Pillai, LS & Kumar, D 2020, ‘Seasonal variations in the diversity and abundance of butterflies in the forest of Champaner-Pavagadh, Gujarat’, Environment and Ecology (Kalyani), vol. 38, no. 1, pp. 62-70.
PIRSA 2019, Plant quarantine standard: South Australia, v14.1 March 2019, Primary Industries and Regions, South Australia, available at http://www.pir.sa.gov.au/biosecurity/plant_health.
Potkar, VR & Jadhav, PS 2015, ‘Phylogenetic analysis and predicted secondary structure of 5.8S gene in Leptosphaerulina trifolii’, International Journal of Advances in Pharmacy, Biology and Chemistry, vol. 4, no. 2, pp. 336-41.
Prakash, PY, Seetaramaiah, VK, Thomas, J, Khanna, V & Rao, SP 2012, ‘Renal fungal bezoar owing to Geotrichum candidum’, Medical Mycology Case Reports, vol. 1, no. 1, pp. 63-5.
Pushpaveni, G, Rao, PRM & Rao, PA 1974, ‘Occurrence of Cerococcus hibisci Green on Hibiscus rosasinensis Linn. in Andhra Pradesh’, Indian Journal of Entomology, vol. 35, no. 1, p. 73. (Abstract only)
QDAF 2023, ‘Vegetable leaf miner’, Queensland Government Department of Agriculture and Fisheries (QDAF), Brisbane, Australia, available at Https://www.business.qld.gov.au/industries/farms-fishing-forestry/agriculture/biosecurity/plants/priority-pest-disease.
Rajgopal, NN & Srinivasa, N 2017, ‘Comparative infestation of red spider mite, Tetranychus macfarlanei and abundance of phytoseiid predator, Neoseiulus longispinosus on okra germplasms across growing seasons under Bangalore conditions’, Journal of Entomology and Zoology Studies, vol. 5, no. 6, pp. 1846-50.
Ramasubbaiah, K & Lal, R 1976, ‘Studies on residues of phosphamidon in okra crop’, Indian Journal of Entomology, vol. 36, no. 4, pp. 344-51. (Abstract only)
Razowski, J 1977, ‘Monograph of the genus Archips Hübner (Lepidoptera, Tortricidae)’, Acta Zoologica Cracoviensia, vol. 30, no. 9, pp. 55-206.
Reddy, J 2019a, Growing okra from seed (bendakaya); bhendi planting, Gardening Tips, India, available at https://gardeningtips.in/growing-okra-bendakaya-planting-care-harvesting.
Robinson, GS, Ackery, PR, Kitching, IJ, Beccaloni, GW & Hernández, LM 2022, ‘HOSTS: a Database of the World's Lepidopteran Hostplants’, Natural History Museum, London, United Kingdom, available at https://www.nhm.ac.uk/our-science/data/hostplants/search/index.dsml, accessed 2022.
Rolania, K, Yadav, SS & Saini, RK 2016, ‘Evaluation of insecticides against blister beetle (Mylabris pustulata Thunb.) on pigeonpea, Cajanus cajan’, Journal of Applied and Natural Science, vol. 8, no. 1, pp. 97-9.
Sahayaraj, K 2015, ‘Entomopathogens for cotton defoliators management’, in Biocontrol of lepidopteran pests, Sree, SK & Varma, A (eds), Springer International Publishing, Switzerland.
Sakthivel, P, Karuppuchamy, P, Kalyanasundaram, M & Srinivasan, T 2012, ‘Host plants of invasive papaya mealybug, Paracoccus marginatus (Williams and Granara de Willink) in Tamil Nadu’, Madras Agricultural Journal, vol. 99, no. 7-9, pp. 615-9.
Sarada, G, Manjula, K, Muralikrishna, T, Gopal, K, Reddy, BR & Nagaraju, R 2020, ‘Screening of muskmelon genotypes aganist melon fruit fly, Zeugodacus cucurbitae (Coquillett) under field conditions’, Journal of Pharmacognosy and Phytochemistry, vol. 9, no. 3, pp. 57-62.
Saranraj, P, Sivasakthivelan, P & Sivasakti, S 2016, ‘Prevalence of fungal diseases in medicinal plants of Vellore district of Tamil Nadu in India’, International Journal of Advanced Multidisciplinary Research, vol. 3, no. 12, pp. 49-63.
Savitri, M, Kumar, VK, Kumari, A, Angmo, K & Bhalla, TC 2017, ‘Isolation and characterization of lactic acid bacteria from traditional pickles of Himachal Pradesh, India’, Journal of Food Science and Technology, vol. 54, no. 7, pp. 1945-52.
Schwinghamer, MW, Schilg, MA, Walsh, JA, Bambach, RW, Cossu, RM, Bambridge, JM, Hind-Lanoiselet, TL, McCorkell, BE & Cross, P 2014, ‘Turnip mosaic virus: potential for crop losses in the grain belt of New South Wales, Australia’, Australasian Plant Pathology, vol. 43, pp. 663-78.
Sharma, D & Rao, DV 2012, ‘A field study of pest of cauliflower, cabbage and okra in some areas of Jaipur’, International Journal of Life Sciences Biotechnology and Pharma Research, vol. 1, no. 2, pp. 122-7.
Sharma, G, Kumar, R, Pathania, PC & Ramamurthy, VV 2008, ‘Biodiversity of lepidopterous insects associated with vegetables in India - a study’, Indian Journal of Entomology, vol. 70, no. 4, pp. 369-84.
Shivas, RG 1989, ‘Fungal and bacterial diseases of plants in Western Australia’, Journal of the Royal Society of Western Australia, vol. 72, no. 1-2, pp. 1-62.
Shivas, RG, Tan, YP, Edwards, J, Dinh, Q, Maxwell, A, Andjic, V, Liberato, JR, Anderson, C, Beasley, DR, Bransgrove, K, Coates, LM, Cowan, K, Daniel, R, Dean, JR, Lomavatu, MF, Mercado-Escueta, D, Mitchell, RW, Thangavel, R, Tran-Nguyen, LTT & Weir, BS 2016, ‘Colletotrichum species in Australia’, Australasian Plant Pathology, vol. 45, no. 5, pp. 447-64.
Singh, AP, Singh, NB & Singh, R 1973, ‘Relationship between different host plants; potential growth and longevity of Thalassodes quadraria Gruen. (Lepidoptera: Geometridae)’, Agra University Journal of Research, vol. 22, no. 3, pp. 55-60.
Singh, G 2012, Checklist of commercial varieties of vegetables, Department of Agriculture and Cooperation, New Delhi, India.
Singh, V & Chauhan, U 2019, ‘First report of red vegetable mite, Tetranychus neocaledonicus Andre (Acari: Tetranychidae) on apple (Malus domestica Borkh) from India’, Journal of Entomology and Zoology Studies, vol. 7, no. 4, pp. 436-8.
Singh, VK & Narain, U 2008, ‘Host range studies of Myrothecium roridum causing leaf spot in grapevine’, Research on Crops, vol. 9, no. 1, pp. 178-80.
Smart Gardener 2019, ‘Okra Rust’, available at https://www.smartgardener.com/plants/7462-okra-okra/diseases/579-rust.
Smetacek, P 2008, ‘Moths recorded from different elevations in Nainital district, Kumon Himalaya, India’, BIONOTES, vol. 10, no. 1, pp. 1-26.
Southgate, BJ 1979, ‘Biology of the Bruchidae’, Annual Review of Entomology, vol. 24, pp. 449-73.
Srinivasan, G & Prabakar, D 2013, A pictorial handbook on grasshoppers of Western Himalayas, Director, Zoological Survey of India, Kolkata, India.
Sushil, SN, Singh, JP, Kapoor, KS & Chakraborty, A 2020, ‘Integrated pest management (IPM) in okra (Abelmoschus esculentus) for export purpose’, Government of India, Ministry of Agriculture & Farmers Welfare, Haryana, India, available at http://ppqs.gov.in/publication.
Syed, RA, Ghani, MA & Murtaza, M 1970, ‘Studies on trypetids and their natural enemies in West Pakistan. III. Dacus (Strumeta) zonatus (Saunders)’, Technical Bulletin, Commonwealth Institute of Biological Control, vol. 13, pp. 1-16.
Tan, DC, Flematti, GR, Ghisalberti, EL, Sivasithamparam, K, Chakraborty, S, Obanor, F & Barbetti, MJ 2011, ‘Mycotoxins produced by Fusarium species associated with annual legume pastures and ‘sheep feed refusal disorders’ in Western Australia’, Mycotoxin research, vol. 27, pp. 123-35.
Tara, J, Sharma, S & Kour, R 2010, ‘A record of weevil (Coleoptera: Curculionoidea) diversity from district Samba (J & K)’, The Bioscan, vol. 5, no. 3, pp. 391-4.
Tindall, HD 1987, Vegetables in the Tropics (Macmillan International College Edition), Tindall, HD (ed), Macmillan Press Limited, London.
TNAU-NAIP 2011, Origin, area, production, varieties, package of practices for bhendi (syn: Lady's finger, Bhindi) (Abelmoschus esculentus (L.) Moench) (2n=130), Tamil Nadu Agricultural University (TNAU) and National Agricultural Innovation Project (NAIP), Coimbatore, India, available at http://eagri.org/eagri50/HORT281/lec06.html.
Tuskegee University 2009, Identification of appropriate postharvest technologies for improving market access and incomes for small horticultural farmers in Sub-Saharan Africa and South Asia, Tuskegee, Alabama, USA, available at https://www.tuskegee.edu/Content/Uploads/Tuskegee/files/CAENS/Others/Post%20Harvest/Assign4/CSA-OKRA%20India.pdf (pdf 1 mb).
Tyagi, K & Kumar, V 2014, ‘New records of thrips (Thysanoptera, Terebrantia, Thripidae) from Himachal Pradesh, India’, Records of the Zoological Survey of India, vol. 114, no. 4, pp. 591-8.
Vantika Tech 2020, ‘Improved varieties of okra (bhindi)’, Vantika Tech, Delhi, India, available at https://www.vantikatech.com/2020/02/improved-varieties-of-okra-bhindi.html.
Varga, J, Kocsubé, S, Tóth, B, Frisvad, JC, Perrone, G, Susca, A, Meijer, M & Samson, RA 2007, ‘Aspergillus brasiliensis sp. nov., a biseriate black Aspergillus species with world-wide distribution’, International Journal of Systematic and Evolutionary Microbiology, vol. 57, no. 8, pp. 1925-32.
Vawdrey, LL 2001, ‘Quantification of inoculum density of Phytophthora palmivora in soil and its relation to disease incidence in papaw in far northern Queensland’, Australasian Plant Pathology, vol. 30, no. 3, pp. 199-204.
Vedham, K, Kolatkar, MD & Muralirangan, MC 2002, ‘Effects of abiotic factors on the population of an acridid grasshopper, Diabolocatantops pinguis (Orthoptera: Acrididae) at two sites in southern India: a three-year study’, Journal of Orthoptera Research, vol. 11, no. 1, pp. 55-62.
Venkataravanappa, V, Reddy, CNL, Jalali, S & Reddy, MK 2013, ‘Molecular characterization of a new species of begomovirus associated with yellow vein mosaic of bhendi (okra) in Bhubhaneswar, India’, European Journal of Plant Pathology, vol. 136, pp. 811-2.
Venkatesha, Gopinath, VK & Chandramohan, K 1992, ‘New host record and some parasitoids of Euproctis fraterna (Moore) (Lepidoptera: Lymantriidae), a pest of forest trees in India’, Indian Journal of Forestry, vol. 15, no. 4, p. 358. (Abstract only)
Virgilio, M, Jordaens, K, Verwimp, C, White, I & De Meyer, M 2015, ‘Higher phylogeny of frugivorous flies (Diptera,Tephritidae,Dacini): localised partition conflicts and a novel generic classification’, Molecular Phylogenetics and Evolution, vol. 85, pp. 171-9.
Visagie, CM, Hirooka, Y, Tanney, JB, Whitfield, E, Mwange, K, Meijer, M, Amend, AS, Seifert, KA & Samson, RA 2014, ‘Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world’, Studies in Mycology, vol. 78, pp. 63-139.
Walter, DE, Halliday, RB & Smith, D 1995, ‘The Oriental red mite, Eutetranychus orientalis (Klein) (Acarina: Tetranychidae), in Australia’, Journal of the Australian Entomological Society, vol. 34, no. 4, pp. 307-8.
Wang, H, Bai, Y, Li, G, Luo, J & Li, C 2020, ‘Characterization of the complete mitochondrial genome of Aulacophora indica (Insecta: Coleoptera: Chrysomeloidea) from Zhijiang’, Mitochondrial DNA Part B, vol. 5, no. 2, pp. 1459-60.
Weinert, MP, Smith, BN, Wagels, G, Hutton, D & Drenth, A 1998, ‘First record of Phytophthora capsici from Queensland’, Australasian Plant Pathology, vol. 28, p. 93.
White, IM & Elson-Harris, MM 1994, Fruit flies of economic significance: their identification and bionomics, CAB International and ACIAR, Wallingford, UK.
Yadav, Y, Maurya, PK, Devi, AP, Jamir, I, Bhattacharjee, T, Banerjee, S, Dutta, S, Debnath, D, Mandal, AK, Dutta, S & Chattopadhyay, A 2018, ‘Enation leaf curl virus (ELCV): a real threat in major okra production belts of India: a review’, Journal of Pharmacognosy and Phytochemistry, vol. 7, no. 2, pp. 3795-802.
Young, GR & Zhang, L 1998, ‘IPM of melon thrips, Thrips palmi Karny (Thysanoptera: Thripidae), on eggplant in the top end of the Northern Territory’, Proceedings of the Sixth Workshop for Tropical Agricultural Entomologists, Darwin, Australia, 11-15 May 1998, Department of Primary Industry and Fisheries, Darwin, pp. 101-11.
Zingore, KM, Sithole, G, Abdel-Rahman, EM, Mohamed, SA, Ekesi, S, Tanga, CM & Mahmoud, MEE 2020, ‘Global risk of invasion by Bactrocera zonata: implications on horticultural crop production under changing climatic conditions’, PLoS ONE, vol. 15, no. 12, e0243047, https://doi.org/10.1371/journal.pone.0243047.
Zobel, ES, Hooks, CR & Dively, GP 2016, ‘Seasonal abundance, host suitability, and feeding injury of the brown marmorated stink bug, Halyomorpha halys (Heteroptera: Penatomidae), in selected vegetables’, Journal of Economic Entomology, vol. 109, no. 3, pp. 1289-302.





