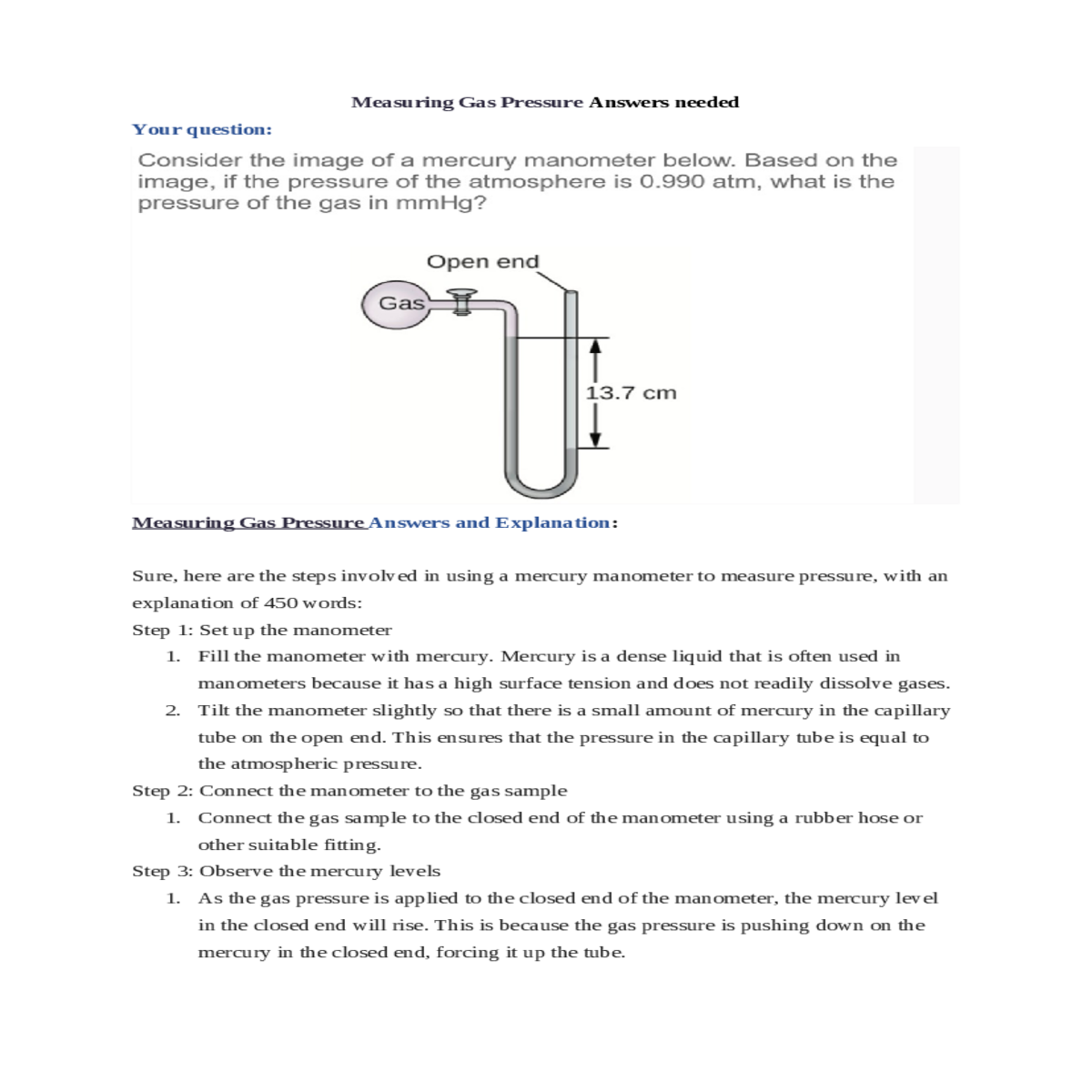
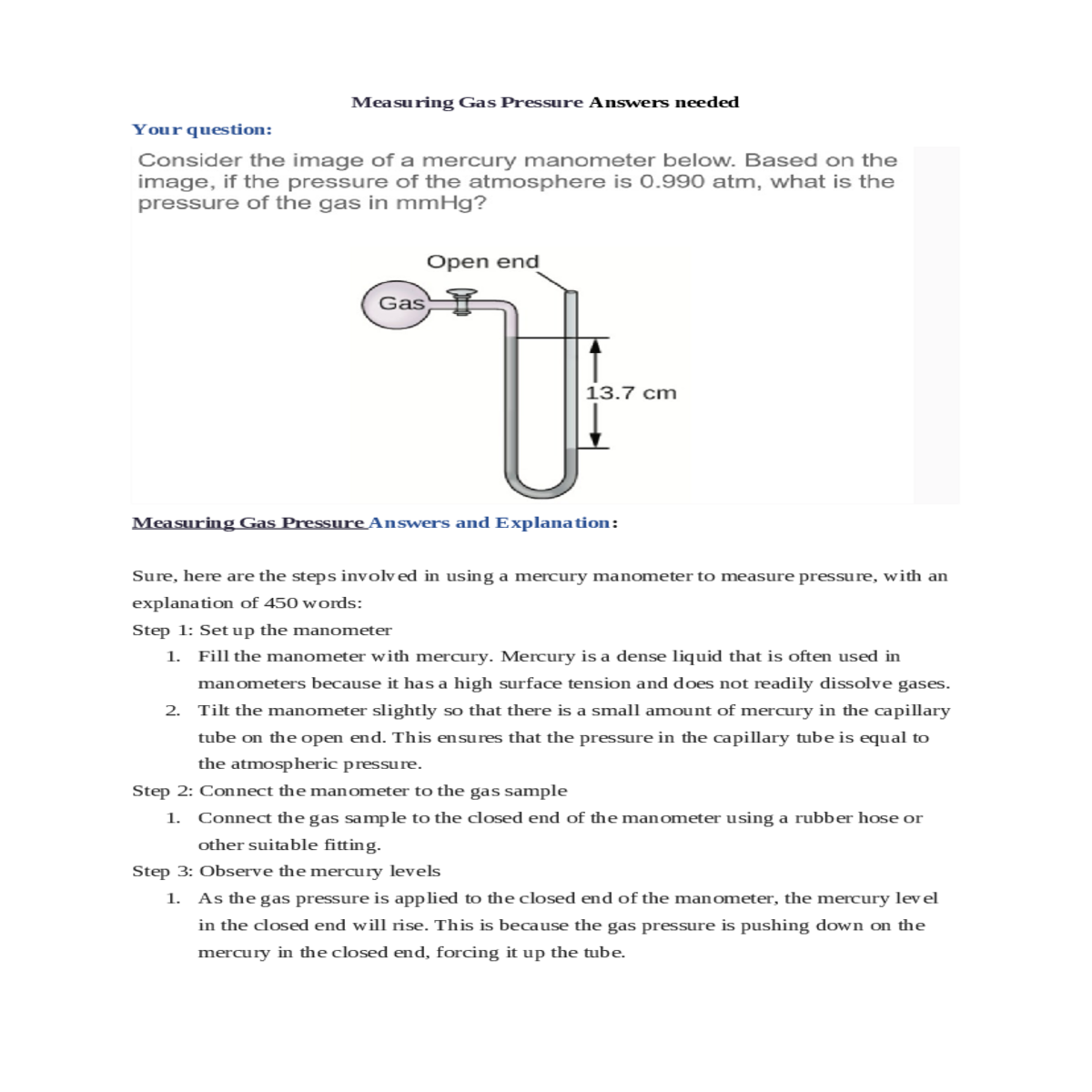
Measuring Gas Pressure Answers needed
Your question:
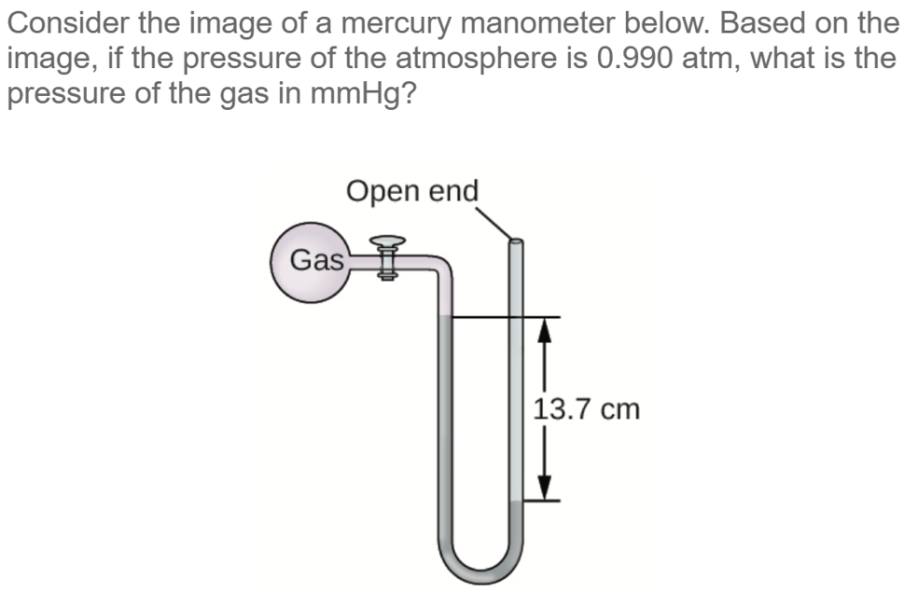
Measuring Gas Pressure Answers and Explanation:
Sure, here are the steps involved in using a mercury manometer to measure pressure, with an explanation of 450 words:
Connect the gas sample to the closed end of the manometer using a rubber hose or other suitable fitting.
Step 3: Observe the mercury levels
The pressure of the gas sample can be calculated using the following equation:
Pgas = Patm - h
For example, if the pressure of the atmosphere is 760 mmHg and the difference in the height of the mercury columns is 100 mm, then the pressure of the gas sample is 660 mmHg.
Additional notes: