Explain what the term impure means element and can pure
|
|---|




A This ‘ice hotel’ is made entirely from ice and snow – these are both water in
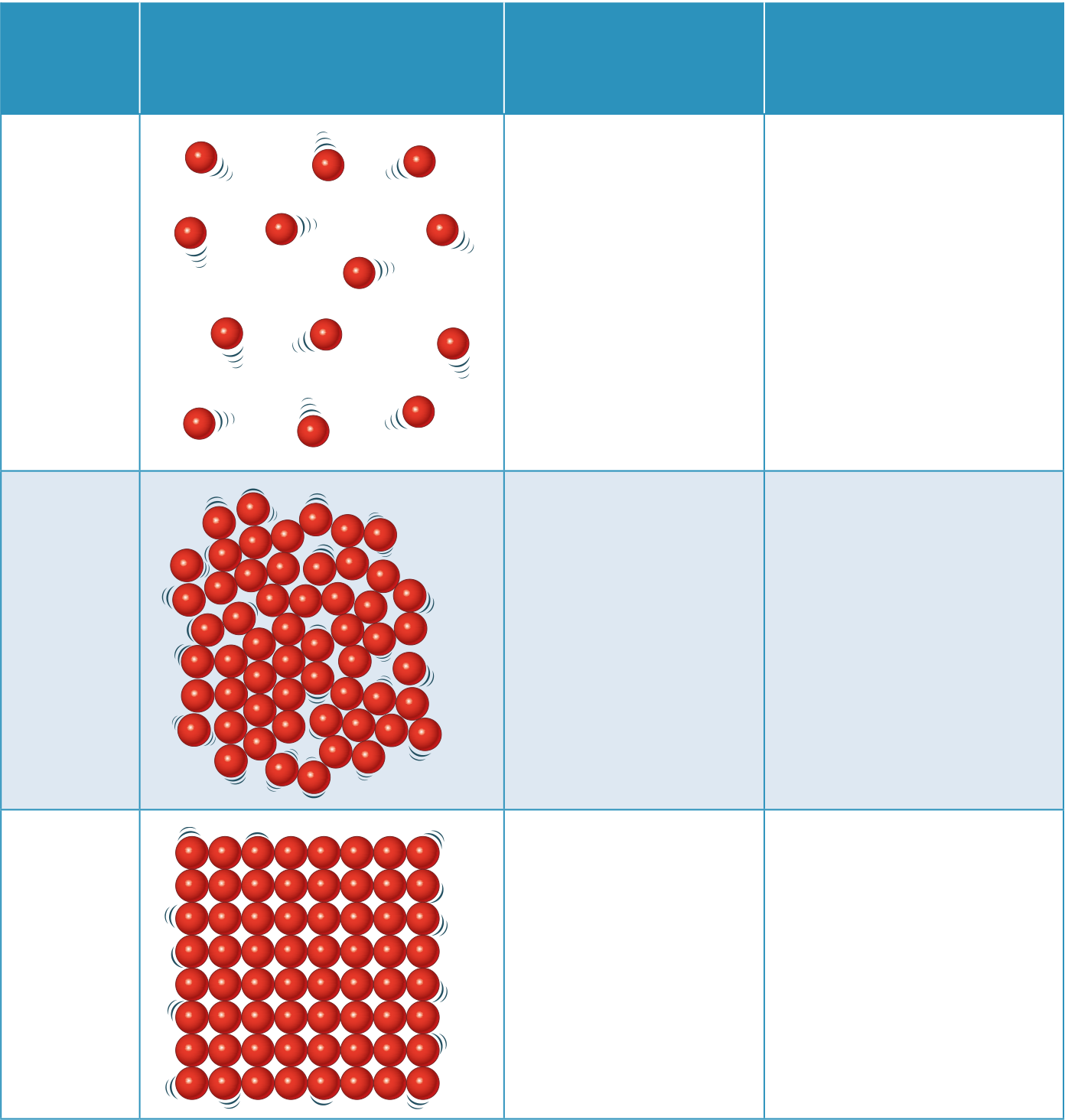
| State |
|
|
|
|---|---|---|---|
| of particles |
| Gas | random | ||
|---|---|---|---|
| far apart |
|
Did you know?
| Liquid | random | |||
|---|---|---|---|---|
|
||||
| close together |
|
|||
| 6 | Solid |
|
regular |
|
|
|---|---|---|---|---|---|
| close together |
B Particles in the solid state contain the smallest amount of stored energy; particles solid
in the gas state contain the most.
| State changes | evaporation | gas | ||
|---|---|---|---|---|
| State changes are physical changes. They can be reversed, and the | ||||
| chemical properties of the substance do not change. This is because | ||||
| the particles themselves do not change – only their arrangement, | ||||
| and boiling | ||||
| movement and amount of stored energy. | ||||
| 2 |
|
|
liquid | deposition |
| solid | ||||
| melting | ||||
| Particles are attracted to one another by weak forces of attraction. | ||||
| There are many of these forces in a solid. Some of these are overcome | ||||
| during melting. The remaining attractive forces between particles | ||||
| in a liquid are overcome during evaporation and boiling (when a | ||||
| Diagram D shows how the temperature changes when water in the solid state is heated until it reaches the gas state. | 5 |
|
|||
|---|---|---|---|---|---|
|
|||||
| Some attractive forces form between particles | |||||
| during condensing, and many attractive forces are formed during freezing. | 7 | ||||
| For this to happen, energy |
|
||||
| must be transferred |
|
6 | |||
| from the particles to the | Temperature (°C) | liquid |
|
||
| surroundings. This is why | |||||
| water vapour turns into | |||||
| water droplets on a cold | |||||
| window, and why you | |||||
| put water in a freezer to | |||||
| make ice. | |||||
| You can predict the state | solid |
|
|||
|
|||||
| of a substance if you know | |||||
| its temperature, and its melting point and boiling point. If the temperature is: |
|||||
Exam-style question Extend
Exam-style questions will follow on publication of the sample assessment materials by Edexcel.




















|
|---|
The composition (make-up) of a pure substance:
• cannot be changed
Gold is an element and can be pure, but
compounds can also be pure. The sugar we use
at home is a compound called sucrose. It containsA You can tell this gold bar is very nearly pure because of the ‘999.9’ stamped on it. A number lower than 1000 on this ‘fineness’ scale means it is impure.
B Pure sucrose is always sucrose, no matter how finely it is ground down.
| 5 | 3a Describe what a mixture | |
|---|---|---|
| 4 | ||
|
||
|
||
| 5 | ||
|
||
|
||
| Explain why this would | ||
|
||
|
Melting points
When a solid melts, its particles gain enough energy to overcome the weak forces of attraction between them. They move further away from one another and the solid becomes a liquid. The temperature at which this
| 5 | ||
|---|---|---|
A pure substance has the same composition in every part of it, and so its physical properties are the same in every part. So, all of a pure substance will melt at the same temperature until all the substance has changed state. The melting point of pure gold is 1063 °C and the melting point of oxygen is −218 °C.

| Temperature (°C) |
|---|
Time (seconds)
D heating curves for a pure substance and a mixture
|
|
|
|
| 5 |
|
|
|---|---|---|
| a Identify which substances are mixtures and which are pure. | ||
| 7 | b Sketch a cooling curve for each of the three examples and | |
|
|
|---|
E1 A piece of gold jewellery is 750 on the fineness scale. Would you expect the jewellery to have a sharp melting temperature? Explain your answer.
|
|---|
• How can crystallisation be used to separate mixtures?
• What are the hazards and risks when separating mixtures by filtration and crystallisation?
They open their mouths and take in water. When they close their mouths, they push out the water through filters. Small animals (such as krill) get stuck in the filters and are swallowed.
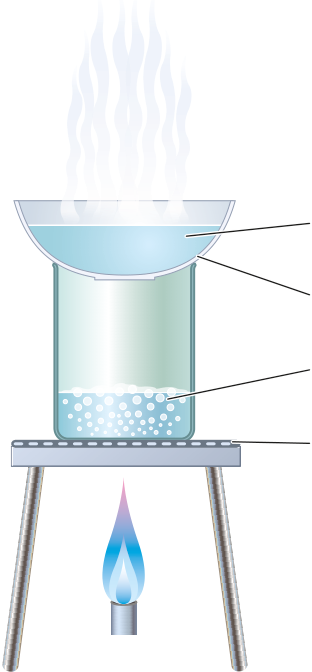
Crystallisation
| 4 | ||
|---|---|---|
|
separated by filtration.
B Crystals in the Giant Crystal Cave in Mexico took over 500 000 years to form.





















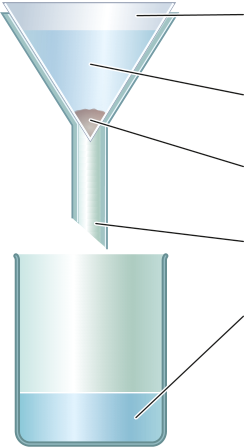
Filtration and crystallisation
| 4 |
|---|
3 In the solution mining of salt, give the names of the:
|
5 | |||
|---|---|---|---|---|
| 5 | ||||
| i | ii | |||
| water vapour | 5 | |||
| 5 |
|
|||
 |
|
|||
| 5 |
|
|||
|
||||
| 6 |
|
|||
|
||||
During crystallisation, the risks from spitting can be reduced by wearing eye previous page?
protection, removing the Bunsen burner before the solution is completely dry and/or using steam to heat the evaporating basin gently (as above).
| 5 | ||
|---|---|---|
|
|
|
| 5 | ||
| 4 |
|
|
| 5 | This solvent dissolves many | |
|
||
| plant compounds. However, | ||
| methanol is flammable and |
25
toxic (especially if the vapour is inhaled). Large crystals can be made to help scientists
work out what the compounds are made of. Explain how you would make plant-compound crystals using methanol.
• How can chromatography be used to separate mixtures?
• What are the differences between mixtures and pure substances on a chromatogram?• How do you calculate an Rf value?
| 5 |
|
|
X | Y | ||
|---|---|---|---|---|---|---|
| compounds are in | ||||||
|
||||||
|
||||||
| 6 | b For mixture Y, explain | paper | ||||
| diferent extents in the | ||||||
| 5 |
|
|
||||
|
||||||
|
||||||
| 5 |
|
|
||||
| B paper chromatography | ||||||
are above the level of solvent in the container.
The Rf value is the distance the compound has risen divided by the distance the solvent has risen. Both measurements are made from the starting
| 6 | ||
|---|---|---|
|
||
|
||





















Paper chromatography
| 6 | |||
|---|---|---|---|
|
|||
|
|||
| the Rf value of the yellow | |||
| value bigger than 1, you’ve made a mistake. |
|
||
Paper chromatography can be used to:
| • distinguish between pure and impure substances | 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| • identify substances by comparing the pattern on the chromatogram with | ||||||||||
|
||||||||||
|
||||||||||
| • identify substances by calculating their Rf values. | ||||||||||
 |
 |
 |
5 | |||||||
 |
 |
|||||||||
| 6 | ||||||||||
|
||||||||||
 |
 |
 |
 |
 |
 |
 |
||||
 |
 |
 |
the most soluble dye? | |||||||
 |
 |
|||||||||
| E104 E110 E120 E122 E133 |
|
|||||||||
C The chromatogram on the left was done using known substances. The chromatogram on the right shows that the orange and blue sweets contain single dyes.
|
||
|---|---|---|
|
||
| D Chromatography can be used to help | ||
|
four orange lipsticks from | |
|
||
|
||
|
||
27
Extend
these Rf values to be useful.
|
|---|
• How do simple distillation and fractional distillation differ?
• How would you reduce risks when carrying out a distillation experiment?
6 b Explain why some irons may not work well if you use ordinary tap water.
When mineral water evaporates, only the water turns to a gas (vapour). The solid minerals, which have much higher boiling points, are left behind.
| 4 | conical | |||
|---|---|---|---|---|
|
||||
|
||||
| tube | ||||
| 5 | 3 Suggest a way of improving | flask | ||
|
|
|||
flask and the vapour travels along the
|
|||||
|---|---|---|---|---|---|
|
|||||
|
|
||||
| 6 |
|
||||
can be used to purify water.
| 6 | |||
|---|---|---|---|
| a the condenser reduces the risk of |
|
6 |
|
|---|
|
||||
|---|---|---|---|---|
| cooling water out |
||||
|
||||
|
||||
• to make alcoholic drinks such as whisky and vodka
hot vapour rises up the column. At first, the vapour condenses when it hits the cool glass and drips back
D distillation apparatus with a fractionating column
|
||
|---|---|---|
| 8 | ||
| 9 | ||
The vacuum flask that we now use to keep drinks hot was originally used
S2 Explain the safety precautions you need to take when carrying out distillation in a laboratory.
|
|---|
29
|
|---|
Progression questions
Purifying sea water
Water is separated from dissolved salts using simple distillation. Sea water is heated so that water vapour leaves it quickly. This vapour is then cooled and condensed, forming water without the dissolved salts.
| 5 | pure water out | ||
|---|---|---|---|
| distillation of sea water |
may be used to provide
drinking water in oil-rich
coastal countries. oil in
| C A cloudy white precipitate forming | 5 |
|
|---|---|---|
|
Water for drinking
In the UK, the raw material for producing drinking water comes from rivers,
| 4 |
|
|---|
• small insoluble particles such as grit and silt
• soluble substances, including salts, pesticides and fertilisersOnly about 2.5% of the Earth’s water is fresh water. Of that, only
|
sand | ||||
|---|---|---|---|---|---|
| rest is in icecaps, glaciers and | |||||
|
|
||||
| stored in tower | |||||
| tank | |||||
gravel
water for
homes and
industry
| 4 | 5 a Describe how water is treated to deal with leaves and twigs, grit | |
|---|---|---|
|
||
| 5 |
|
|
| two ways in which water can | ||
| 6 |
|
|
|
||
|
|---|










