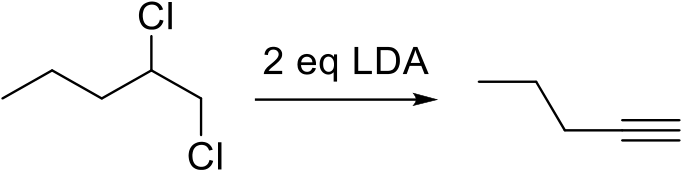
Draw conformers the cis and trans isomers dibromocyclohexane
lOMoARcPSD|24389855

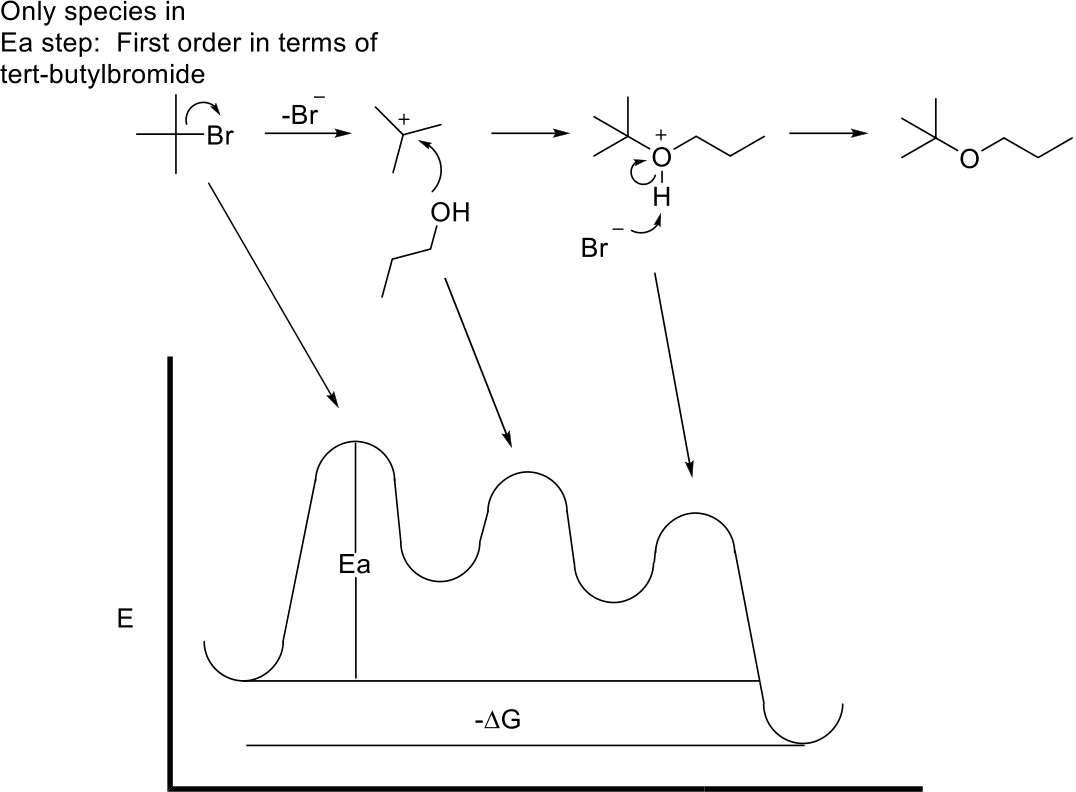
2.Arrange the following molecules in order of increasing energy. (3 pts)
3.Convert the Newman projections into chair conformers. Which is the lower energy conformer and why? (8 pts)
4.Using chair conformations, draw conformers of the cis and trans isomers of 1,2-
dibromocyclohexane. Which of the cis conformers is higher in energy, which of the trans? Why?(10 pts)
The conformer with both bromines in the equatorial position (right) is more favored that both in the
axial position.
Downloaded by bfg gdfg (ANNA44566585@outlook.com)
lOMoARcPSD|24389855
R absolute configuration: The atoms about a chiral carbon are arranged in a clockwise arrangement.
S absolute configuration: The atoms about a chiral carbon are arranged in a counter-clockwise
which are diastereomers. (8 pts)
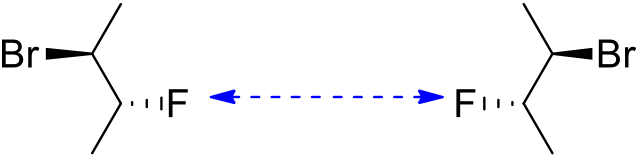
9.Draw the stereoisomers of 1,2-difluorocyclobutane. Indicate which are enantiomers and which
10.What physical/chemical properties do the following sets of stereoisomers have in common? (6 pts)
|
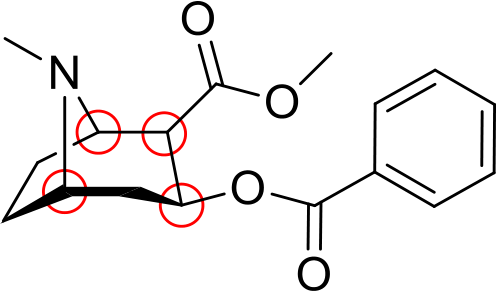
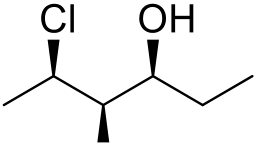
12.Name the following compounds. (indicate any stereochemistry) (4 pts each)
|
|
Weakest | Strongest |
|---|
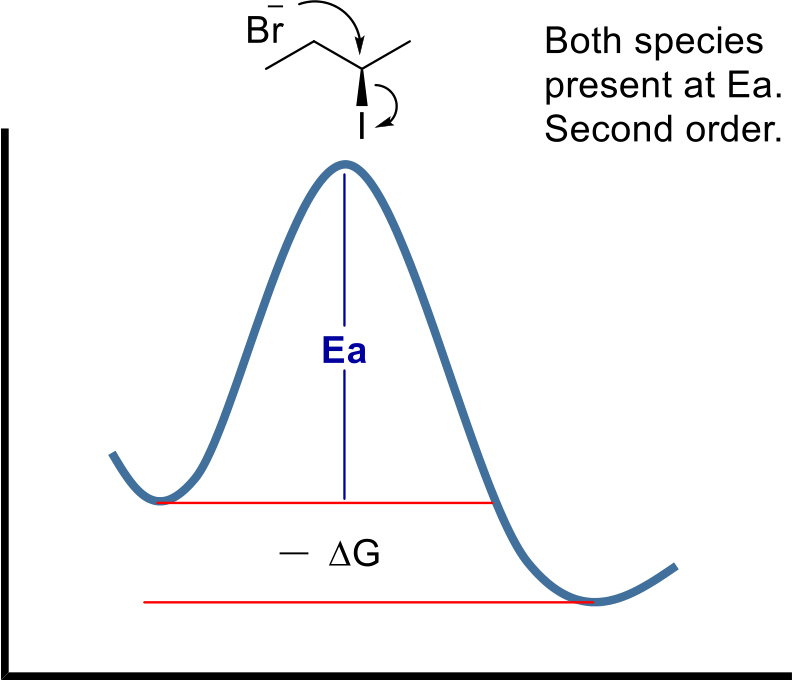
Heterolytic: One species gets both electrons from the bond. In this case a cation and anion is formed.
ΔS is negative. Two molecules reacted to form one. ΔH is very negative. Three pi are broken to form