Spin Selection Rule Explanation Answers needed
Your question:
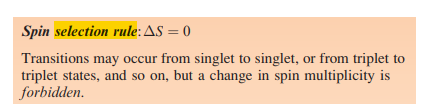
Determining Nonpolar Molecules Answers and Explanation:
Sure, I can do that. The image you sent me shows the spin selection rule, which is one of the basic rules of spectroscopy. The spin selection rule states that the total spin angular momentum of a system must not change during a transition. This means that a molecule or atom can only absorb or emit a photon if the spin of the excited state is the same as the spin of the ground state.
The spin selection rule: The spin selection rule states that the total spin angular momentum of a molecule or atom cannot change during a transition. This means that a molecule or atom can only absorb or emit a photon if the spin of the excited state is the same as the spin of the ground state. For example, a singlet molecule can only absorb or emit a photon if the excited state is also a singlet state. A triplet molecule can only absorb or emit a photon if the excited state is also a triplet state.
Why is the spin selection rule important? The spin selection rule is important because it helps to explain the selection rules for spectroscopic transitions. Selection rules are rules that tell us which transitions are allowed and which transitions are forbidden. The spin selection rule is one of the most important selection rules for electronic spectroscopy.





