Biocide and reagent the synthesis ofsome dyes
6/12/2020 8.3 Module 8 Lesson 3 Assignment: Chemistry 30 (5) Reg - Perrott - 1(A)
8.3 Module 8 Lesson 3 Assignment
| Points 33 | Questions 27 |
|---|
You will be allowed submit this assignment once. Make sure you answer all the questions and save your answers before submitting.
Attempt History
Score for this quiz: 26.67 out of 33
Submitted Jun 12 at 3:04pm
This attempt took 57 minutes.
|
1/26 |
|---|
6/12/2020 8.3 Module 8 Lesson 3 Assignment: Chemistry 30 (5) Reg - Perrott - 1(A)
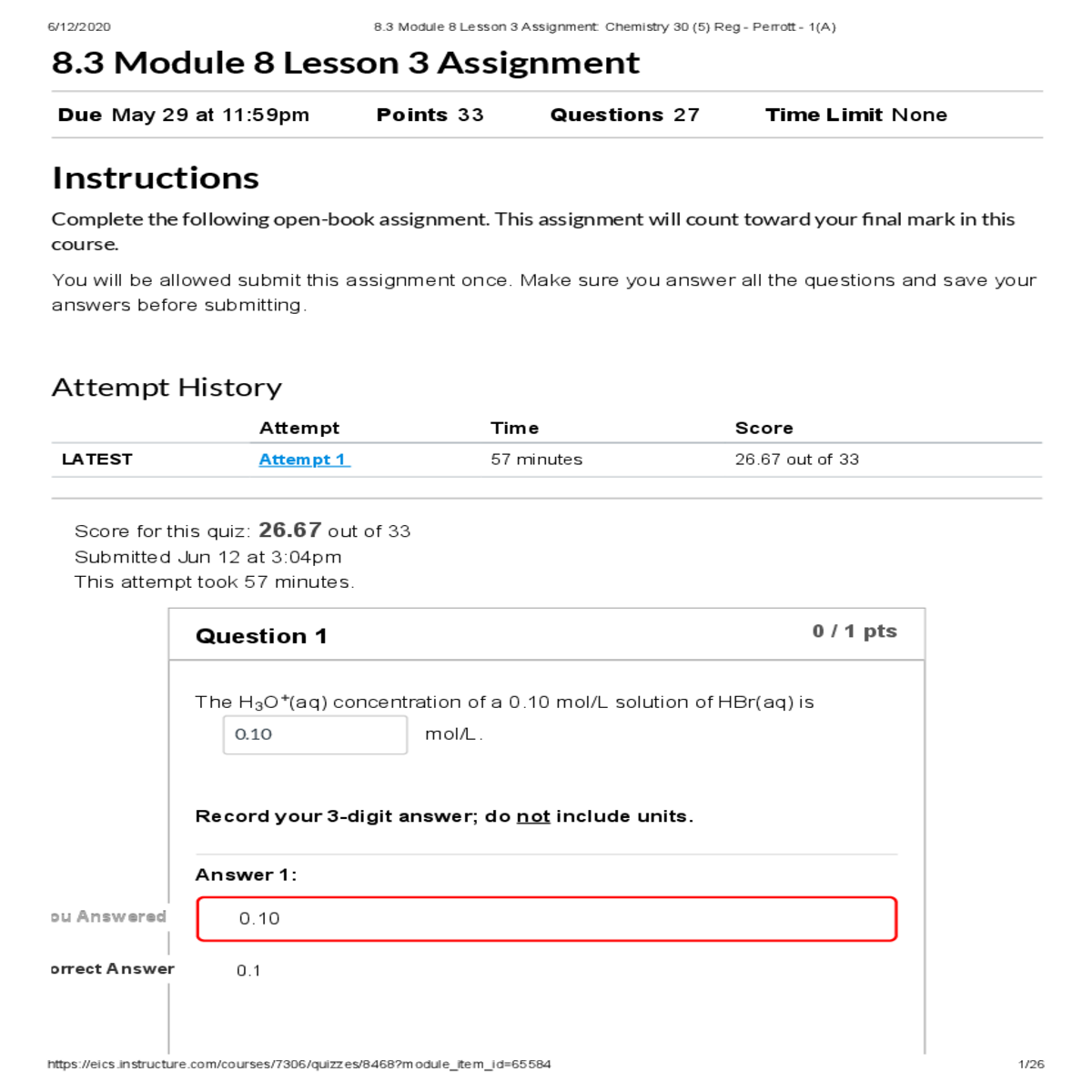
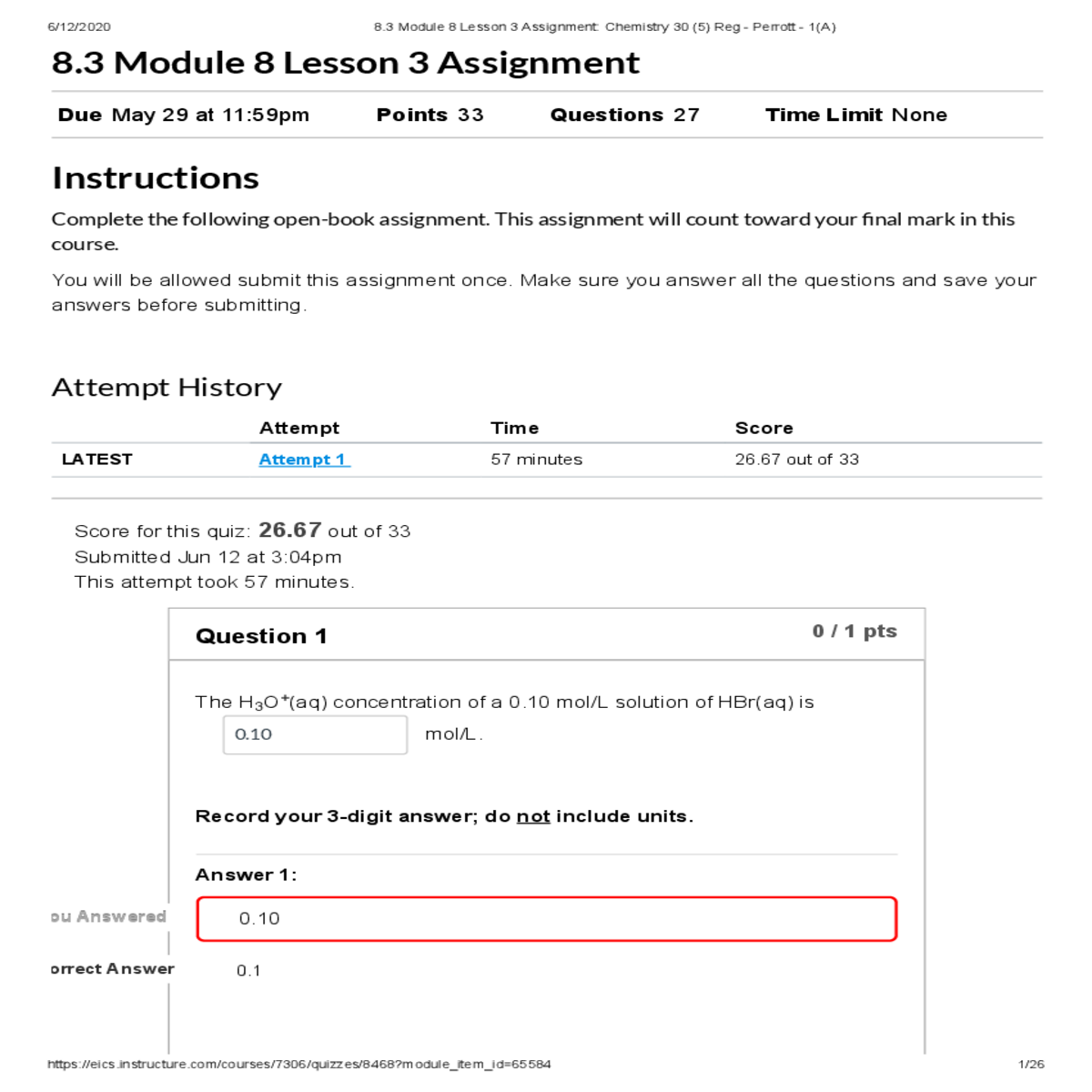
Strong acid so it will ionize 100% [HBr(aq)] = [H O (aq)] 3 +
Answer 1:
Correct! 6
Weak acid - will ionize less than 100%. You need to use the
equilibrium law expression. The acid concentration is more than 1000 times greater than the Ka value, therefore any decrease in concentration due to ionization is considered negligible and the equilibrium concentration of the acid is assumed to be the same as the initial concentration.
| 2/26 |
|---|
Record your 3-digit answer
Answer 1:
6/12/2020 8.3 Module 8 Lesson 3 Assignment: Chemistry 30 (5) Reg - Perrott - 1(A)
This is a weak acid, therefore will not ionize 100%. The acid concentration is more than 1 000 times greater than the K value, a
therefore any decrease in concentration due to ionization is
considered negligible and the equilibrium concentration of the acid is assumed to be the same as the initial concentration
| 4/26 |
|---|
Correct!
| Correct! | 5/26 | |
|---|---|---|
| Correct! | ||
| Correct! |
|
||
|---|---|---|
| Question 8 | 1 / 1 pts | |
|
||
not ionize to any great extent
| 7/26 |
|---|
| notation, is a.b x 10 mol/L. The values of a,b, and c are -c | ||
|---|---|---|
|
||
Answer 1:
|
|---|
Answer 3:
| 6/12/2020 |
|
|
|---|---|---|
| 1 / 1 pts |
4.7×10−11
4.5×10−7
| 8.3 Module 8 Lesson 3 Assignment: Chemistry 30 (5) Reg - Perrott - 1(A) | ||
|---|---|---|
|
1 / 1 pts | |
|
||
Correct! Correct! |
||
|---|---|---|
| 1 / 1 pts | ||
Answer 1:
Correct! higher
6/12/2020 8.3 Module 8 Lesson 3 Assignment: Chemistry 30 (5) Reg - Perrott - 1(A)
Question 14 1 / 1 pts
Correct! 3
Answer 2:
Correct! 2
Kb=Kw/Ka
| 12/26 |
|---|
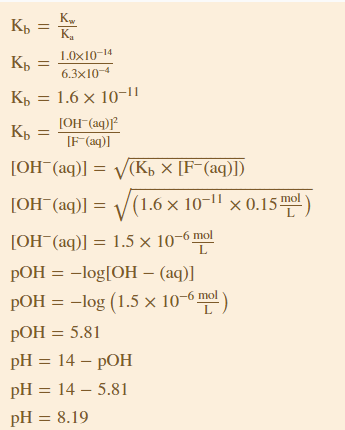

|
15/26 |
|---|
| 8.3 Module 8 Lesson 3 Assignment: Chemistry 30 (5) Reg - Perrott - 1(A) |
|---|
|
|
19/26 |