


MScCH-07 Vardhman Mahaveer Open University, Kota
School of Science & Technology
Vardhman Mahaveer Open University, Kota Prof L.R.
Gurjar
Director Academic
VMOU Kota
Dr. Anuradha Dubey
Deputy Director,SOST
VMOU Kota
Prof. P.S. Verma (Retd.)
Department of Chemistry
University of Raj, Jaipur
Prof. P.D. Sharma (Retd.)
|
|
|
|
|
Dr. Sushil Kumar Sharma
Assistant Professor, Department of Pure and Applied Chemistry
|
University of Kota, Kota
5.7,12
|
9,10
|
| Academic and Administrative
Management |
|
Prof. L.R. Gurjar
|
Vice-Chancellor
Vardhman Mahaveer Open University, Kota Prof. Karan
Singh
|
|
|
|
Printed and Published on behalf of the Registrar, V.M. Open
University, Kota.
Printed by :
| Unit -1 |
Organometallic Reagents-I
|
1
|
| Unit -2 |
|
|
| Unit -3 |
|
|
| Unit -4 |
Oxidation of – OH group
|
53
|
| Unit -5 |
|
|
| Unit -6 |
|
|
| Unit -7 |
Reduction of Carbonyl and Carboxylic Group
|
106
|
| Unit -8 |
|
|
| Unit -9 |
|
|
| Unit -10 |
Nonbenzenoid Aromatic Compounds: -General
consideration, Monocyclic aromatic anions, Synthesis and reactions of
Tropone and Tropolone
|
173
|
| Unit -11 |
|
|
| Unit -12 |
|
|
| Unit -13 |
Disconnections of C-X Group
|
226
|
| Unit -14 |
|
|
| Unit -15 |
|
|
Compounds
|
| Unit -17 |
Two Group C-C Disconnections
|
298 |
| Unit -18 |
|
319 |
| Unit -19 |
|
345 |
| Unit -20 |
|
360 |
MScCH-07
Vardhman Mahaveer Open University, Kota
Unit - 1
Organometallic Reagents-I
1.5.1 Oxidative- addition reaction
1.5.2Carbanion-halide exchange
1.5.3By vinyl halide
1
1.6.3 Addition with ketene and isocyanates
1.10References and Suggested Readings
1.0Principle of organolithium compounds
NMR studies also indicate that methyl lithium retains the tetrameric
solid state structure in solution. The structures of (Li-R)4 units i.e
tetrameric depicted by X-
2
It is an important method for the preparation of simplest
organolithium reagents. The most important alkyl lithium reagent is
butyl lithium. This method involves the oxidation of a metal M by the
addition of a group R-X.
1.1.4 Metal hydrogen exchange reactions
(Metallation)
| LiC2H5 |
+ |
Ph3CH
|
3 |
LiCPh3 |
+ |
C2H6
|
|
| LiAlH4 |
+ |
4CH2=CH2
|
Pressure 100°C |
|
|
1.1.7By reduction of sulphides
More stable carbanion due to OCH3
Organolithium derivatives are extremely reactive (super Grignard
reagent). Organolithium compounds are oxygen and moisture sensitive and
show Lewis acidic character.
|
|
H2O H5C6
R'Li R-N=C-R'Li
|
Bu
|
|
Organolithium compounds are the most versatile reagents in all fields
of chemistry . Thus some of the important applications of these
compounds are as follows:
1.Organolithium compounds are highly reactive nucleophiles and strong
bases due to the presence of strongly polarized Li-C bond.
5.Organolithium compounds can be used as powerful metallating
agents.
1.4 Principle of organomagnesium
halides
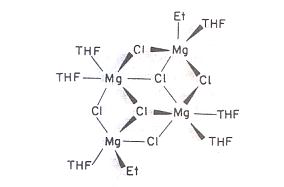
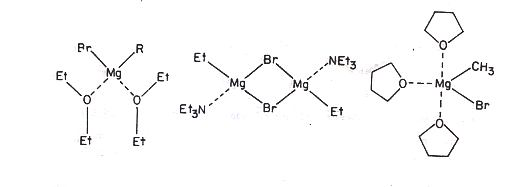
Fig.2 Structure of (a) Mg(Br)R(Et2O)2 , (b) [Mg(Br)Et(NEt3)]2 and
(c)MgBr(Me)(THF)3
Grignard reagents are usually prepared by the action of organic
halides and magnesium turnings in an ether solvent. This is the well
known example of two electron oxidative- addition reaction.
8
1.6Properties of organomagnesium halides
9
| Acid |
base |
Acid |
base |
1.6.2Addition with aldehydes and ketones
Grignard reagent react with carbonyl compounds as shown below:
CH3CHO RMgX / ether
|
1.6.6Nucleophilic substitution reactions at sp2 hybrid
carbon
Grignard reagents react with acid chlorides, esters and lactones to give
alcohols whereas Carbonates react with Grignard reagents to give
esters.
Organomagnesium halides possess a variety of important applications
some of which are as follows:
1.Grignard reagent is one of the most versatile reagents in organic
and organometallic synthesis.
4.Grignard reagent provides a useful method for the preparation of
t-alkyl amines like Me3C-NH2 , as such amines are not obtained from SN2
reaction between t-alkyl halides and ammonia.













