Help with CHROMATOGRAPHY
Chromatography was first described by Tsweet 1903
Principle of Chromatography
The difference in the rate at which the components of a mixture move through porous medium, called stationary phase under the influence of some solvent or gas called moving phase.
TYPES OF CHROMATOGRAPHY
Adsorption chromatography
- Fixed phase: Solid like alumina, magnesium oxide, silica gel.
- Solutes are adsorbed in different parts of column and then eluted by suitable solvent.
Partition chromatography
- Mechanism: Counter current distribution
- Fixed phase: Liquid usually water adsorbed on as solid support.
- Solute gets distributed between adsorbed liquid and moving solvent.
Gas chromatography
- Moving phase: Mixture of gases.
Ion Exchange method of chromatography
- Exchange of ions of like charges between a solution and a solid (a synthetic or natural resin).
PAPER CHROMATOGRAPHY
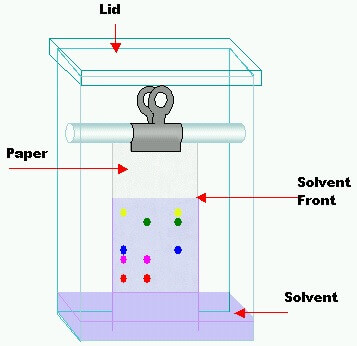
- Introduced by Martin and Synge (1941)
- Paper Chromatography is a special case of partition chromatography
- Solute gets distributed between two liquids
- Paper strip is a solid porous support
- Components migrate at different rates with moving phase
Rf VALUES
Movement of components relative to solvent is expressed in terms of Rf values (Retention Factor).
Rf = Distance travelled by the solute from starting line/ Distance travelled by the solvent from starting line
Rf is characteristic of a species provided the following conditions are same:
- Solvent employed
- Medium used for separation- Quality of paper
- Nature of mixture
- Temperature
- Size of vessel
Factors affecting Rf Values
- Solubility in moving phase- It increases Rf values
- Solvent with donor groups such as –OH, C=O.
- Presence of acids like HCL.
- Nature of counter ion- It decreases Rf values
- Anions form strong water soluble complex.
- Anions forms insoluble precipitate.
- Adsorption is not important when acidic solvents are used
TECHNIQUE OF CHROMATOGRAPHY
- Ascending: The solvent travel up the paper.
- Descending: The solvent travels down the paper.
- Radial: Circular development.
CHOICE OF DEVELOPING SOLVENT
- Suitable polarity
- Donor group
- Acidified
VISUALIZATION
- Chemical reagents forming colored compounds or complexes.
- Sodium Sulphide
- Dithizone (Diphenylthiocarbazone)
- Ph-N=N-C(S)-NH-NH-Ph
- Forms colored metal complexes through two nitrogen and one sulphur atom.
SEPARATIONS
- Cu2+, Cd2+ (Ascending)
- Developing solvent: Ethanol + Conc. HCl (9 parts + 1part)
- Visualising Agent: Na2S
- Spot Colors: Black for Cu2+ Yellow for Cd2+
- Rf Value of Cd2+ > Cu2+
- Ag+, Hg22+(Ascending and radial)
- Developing solvent:
- Butanol + Acetic Acid + water (6parts + 2parts +1part)
- Visualizing Agent: Dithizone
- Spot Colors: Orange for Ag+ Pink for Hg22+
- Rf value of Hg22+ > Ag+
- Pb++, Hg22+ ( Ascending and radial)
- Developing solvent:
- Butanol+ Acetic Acid + Water (6 parts + 2 parts + 1 part)
- Visualing Agent: Dithizone
- Spot Colors: Rose pink for Pb2+ Pink for Hg22+
- Rf value of Hg22+ Pb2+
Inorganic Chromatography
Abstract: Chromatography was first described by Tswett in 1903 for the separation of colored/ mixture substances into individual components. It depends upon the redistribution of the molecules of mixture between two or more phases. Nowadays, various types of chromatography are in use to separate almost any given mixture, whether colored or colorless, into its constituents and to test the purity of these constituents. This technique is based on the difference in the rate at which the components of a mixture move through porous medium (called stationary phase) under the influence of some solvent or gas (called moving phase).
The various types of chromatography include adsorption chromatography, fluid partition chromatography and ion exchange. The main systems employed in partition chromatography are gas partition, liquid partition employing fixed beds (i.e. column chromatography), thin layer and paper chromatography. In liquid partition chromatography a mobile liquid phase flows over an essentially stationary liquid phase absorbed on a support, in paper chromatography the support is paper or treated paper, whereas in thin layer chromatography the adsorbent is coated on a glass plate.
We shall deal only with selected aspects of paper chromatography with special reference to inorganic analysis (referred as inorganic chromatography).
Paper chromatography was introduced by Martin and Synge in 1941. Stationary phase is usually water which is held in the fibers of the paper, moving phase is a solvent or mixture of solvents of desired polarity. In this technique a drop of test solution is applied as a small spot on filter paper and the spot is dried. The paper is place in a close chamber and the edge of the filter paper is then dipped into solvent called developing solvent. The various cations are moved by solvent system at various speeds. The following systems can be separated by this technique and visualized by different reagents.
A. Cu2+ and Cd2+
- Developing solvent: 1. ethanol(9 parts) + HCl conc( 1 part)
- Visualizing agent: Na2S solution
- Spot colors: Black for Cu2+, yellow for Cd2+
B. Ag+, Hg22+, Pb2+
Developing solvent: n-Butyl alcohol mixed with 5%(1/2) of glacial acetic acid followed by water to turbidity or Deionized water
- Visualizing agent: 0.5% Dithizone in CHCl3
- Spot colors: Orange for Ag+
- Pink for Hg22+
- Rose pink for Pb2+
C. Cl-, Br-, I
- Developing solvent: Acetone (8 parts) + water (2 parts)
- Visualizing reagent: 0.1N AgNO3 + Na2S
- Spots: Black
- Rf order Iodide> Bromide> Chloride
D. Fe2+, Co2+, Nc2+, Cu2+
- Developing solvent: Acetone 90ml + 5ml water + 5ml HCl (conc.)
- Visualizing Agent: Rubeanic Acid
List of chemicals and Apparatus
- AgNO3 (10g)
- Hg2(NO3)2 (10g)
- Pb(NO3)2 ( 10g)
- n- Butyl alcohol (500ml)
- Glacial acetic acid (100ml)
- Deionized water (1l)
- Dithiozone (5g)
- Potassium chromate (5g)
- Acetone 500 ml
- Nacl (10g)
- NaBr (10g)
- NaI (10g)
- FeSO4.7H2O (10g)
- CaCl2 (10g)
- NiSO4 (10g)
- CuSO4.5H2O (10g)
- Rubeanic Acid (10g)
- Chloroform (100ml)
- ‘Cu (NO3)2 (10g)
- Cadmium nitrate (10g)
- ethanol (500ml)
- Whatman Filter paper (NO.1) 2 sheets
- Chromatography Jars with lids (10)
- Glass rods
- capillary tubes
- Hydrochloric acid (conc.) 500ml
Need help with mastering chemistry concepts? Looking for help with you Chemistry Assignment? Need Online Chemistry Tutor to explain tough chemistry concepts such as Chromatography, s-block elements, p-block elements, coordination chemistry? Organic Chemistry Assignment due tomorrow? Then Assignmenthelp is the right place to be at. Assignmenthelpnet’s online Chemistry tutors can help you master chemistry concepts and practice important chemistry numerical problems and laboratory chemistry practical. We offer Online Chemistry homework help with Organic Chemistry, Inorganic Chemistry, general Chemistry, Physical Chemistry, Environmental Chemistry or Biochemistry and other allied areas of applied chemistry.


