Properties of Matter Assignment Help
What is matter?
Matter includes everything that we can see around us, like the paper you hold, pencil you use, table, chair etc. But there are two conditions that allow us to designate any substance as a matter:
- Weight
- Space
Yes, matter is anything that have mass/weight and occupies space.
Properties of matter
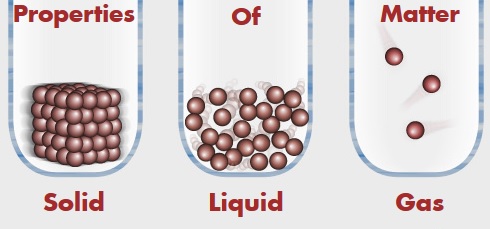
No two substances are similar. Hence, the qualities that distinguish one substance from other are called properties. Matter also have different properties and these properties of matter can be broadly classified into physical properties and chemical properties.
Physical properties:
Physical also denotes appearance. Hence, the difference in appearance of any substance that can be easily viewed by our naked eyes is termed as physical properties. This includes: color, odor, boiling point, texture, solubility, polarity etc. This means that any properties that doesn’t alter the chemical composition of matter is the physical property. This property of matter can again be divided into two main sections: intensive and extensive physical property of matter. Intensive physical property means the property that is independent to the size or quantity of matter; like solubility, color etc. whereas extensive physical property is the property of matter that changes with the change in amount of matter, like the volume, mass and so on.
Properties of Matter Assignment Help Through Online Tutoring and Guided Sessions at MyAssignmentHelp
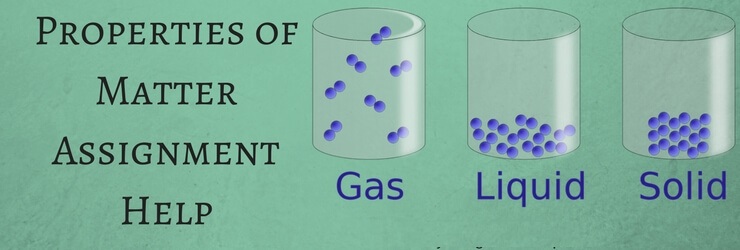
Similarly, Physical property of matter is also connected to physical change. This physical change can be best explained through three states of matter i.e. solid, liquid and gas. In case of physical change; the original structure and appearance of substance can be obtained. Ice is a good example to describe physical change. Melting of ice result in water and heating the water results in water vapor. But this process can be easily reversed by converting water vapor back to water and water back to ice.
Chemical property:
Chemical property reflects the difference in composition of the matter. Chemical property also means that when two similar matters of different chemical component are reacted with same molecule/compound; the output obtain will be different. Hence, the reactivity of matter with different other substances including acid, water, PH, and other chemical helps to determine the chemical property of matter. This can be better explained with an example: There are different types of alcohol available, almost all of these alcohol share at least some common properties like the hydroxyl group attached to a carbon. Hence, when reacted with oxygen i.e. O2 it gives CO2 and H2O.
Matter also undergoes chemical change; which means that once the chemical composition of matter is altered, it is difficult or almost impossible to get back the original compound. Take an example of wood; once the wood is burnt, it gets converted to ash. Now, chemical change doesn’t allow us to get back the original substance that is wood. Hence, these are two important properties of matter that helps to identify the difference between substances.
If you are facing problem with equations or mathematical questions based on matter, visit our site for required help on this topic. We provide Assignment Help on properties of matter, homework help, and project making help from the experienced and well- qualified teachers of this field. If you have any questions, contact our online expert and get direct reply. Our service is 24*7 available, so you can contact us at any point of time.
We provide you the best solutions with accurate explanation. Our teachers will do mathematical calculation for you; providing an appropriate answer. So, rather than piling your work, you can seek help for this topic with our team.


