Ionization of Weak Acids Assignment Help
Ionization of Weak Acids
Let us consider the ionization of weak acid HA, having initial concentration ‘c’ in mol/litre
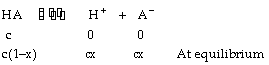

It Ka is ionization constant for acid, then
![]()
![]()
for very weak acids. x < < 1, concentration can be calculated from following formula:
(i) ![]()
(ii) ![]()
(iii) ![]()
Ionization of Weak Acids Assignment Help By Online Tutoring and Guided Sessions at AssignmentHelp.Net
(iv) ![]()
Assignment Help For Ionization of Weak Acids
assignmenthelp.net provides best Online Assignment Help service in Ionization of Weak Acids for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Ionization of Weak Acids assignment click here


