Thermodynamics Computational Problem
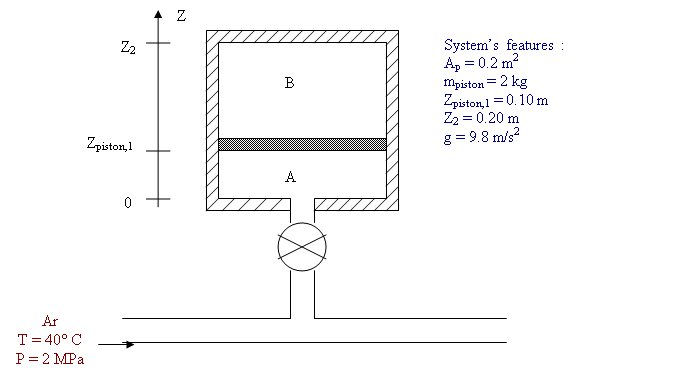
An insulated piston moves freely inside an insulated cylinder as shown in the figure above. Compartment A contains 40 g of argon at 30°C and compartment B contains hydrogen at 20°C. Room A is connected to an argon-supplying line through a valve. The valve is opened until the pressure in compartment A reaches 1 Mpa. The process is slow enough so that the system is in quasi-equilibrium throughout and the gases are considered ideal.
During the process, the volume and the pressure of compartment B are related as follows:
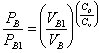
Cp and Cv will be considered constant, equal to their values at standard conditions.
Using the computer language of your choice (MATLAB or Excel recommended), represent the evolution of TA, TB, PA, PB, mA and the work done on the gas in compartment B as a function of Z. Use 100 steps to go from Zinitial to Zfinal. In your volume calculations neglect the thickness of the piston.
Write a report that should as per below:
- The original problem statement
- Detailed description of derivations
- results in the form of graphs
- Discussion of results
Assignment Help | Thermodynamics Assignment Help | Thermodynamics Homework Help | Thermodynamics Project Help | Online Tutoring