Biochemistry of organic functional groups: carbohydrates, lipid, proteins and nucleic acid
Carbohydrates
Carbohydrates are composed of carbon, hydrogen and oxygen and follows the formula (CH2O)n. They can be classified into different groups like Monosaccharides, oligosaccharides and polysaccharides.
Monosaccharide: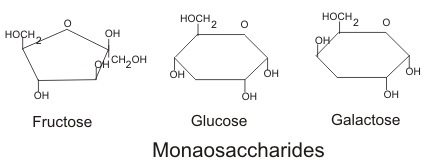 These are also called simple sugars since they have only one sugar molecule and cannot be further hydrolyzed. Based on the functional group they are divided into two types called: aldoses and ketoses. In case of aldoses, the functional group present in the molecule is aldehyde. Example here includes: glucose whereas in case of ketoses the functional group involved is keto group. Example include: fructose. Likewise, based on the number of carbon atoms, carbohydrate can be divided into several categories like trioses( having three sugars), tetroses (having 4 sugars), pentoses (having five sugars) and so on. Image reference: www.flparentingnews.com
These are also called simple sugars since they have only one sugar molecule and cannot be further hydrolyzed. Based on the functional group they are divided into two types called: aldoses and ketoses. In case of aldoses, the functional group present in the molecule is aldehyde. Example here includes: glucose whereas in case of ketoses the functional group involved is keto group. Example include: fructose. Likewise, based on the number of carbon atoms, carbohydrate can be divided into several categories like trioses( having three sugars), tetroses (having 4 sugars), pentoses (having five sugars) and so on. Image reference: www.flparentingnews.com
Disaccharides: It consists of two monosaccharides unit linked together with glycosidic bond. These are of two types: reducing and non- reducing disaccharides. Reducing refers to those that have free aldehyde or keto group and the example include: maltose, lactose. The other is non- reducing Disaccharides having no free aldehyde or keto group. Example include: sucrose.
Maltose: 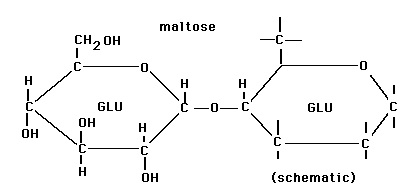 This is reducing sugar that is made of alpha D- glucose linked with other alpha D- glucose by glycosidic bond. Image reference: www.facstaff.gpc.edu
This is reducing sugar that is made of alpha D- glucose linked with other alpha D- glucose by glycosidic bond. Image reference: www.facstaff.gpc.edu
Sucrose: 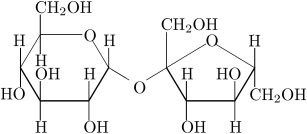 It is non- reducing sugar that is also called cane sugar. It is made of alpha D- glucose and beta D- glucose that are held together by glycosidic bond. A term called inversion is used in case of sucrose that is change in optical rotation from dextrorotatory (+) to levorotatory (-). Therefore, hydrolyzed mixture of sucrose that contains glucose and fructose is called invert sugar which is done by the enzyme the enzyme sucrase. Image reference: www.d.umn.edu
It is non- reducing sugar that is also called cane sugar. It is made of alpha D- glucose and beta D- glucose that are held together by glycosidic bond. A term called inversion is used in case of sucrose that is change in optical rotation from dextrorotatory (+) to levorotatory (-). Therefore, hydrolyzed mixture of sucrose that contains glucose and fructose is called invert sugar which is done by the enzyme the enzyme sucrase. Image reference: www.d.umn.edu
Lactose: 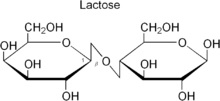 This is reducing sugar that is also called milk sugar. It is made of beta D galactose and beta D glucose held by glycosidic bond. Image reference: www.simple.wikipedia.org
This is reducing sugar that is also called milk sugar. It is made of beta D galactose and beta D glucose held by glycosidic bond. Image reference: www.simple.wikipedia.org
Polysaccharides: 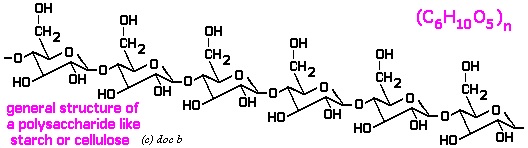 It consists of repeated units of monosaccharides. It can be either linear or the branched polymer. Here linear polymer includes homopolysaccharides that consist of starch, glycogen, cellulose, chitin etc and on the other hand branched polysaccharide is called heteropolysaccharide that include heparin, chondroitin sulphate, hyaluronic acid etc. All these together are termed as polysaccharides that have one or the other function in the living body. Image reference: www.docbrown.info
It consists of repeated units of monosaccharides. It can be either linear or the branched polymer. Here linear polymer includes homopolysaccharides that consist of starch, glycogen, cellulose, chitin etc and on the other hand branched polysaccharide is called heteropolysaccharide that include heparin, chondroitin sulphate, hyaluronic acid etc. All these together are termed as polysaccharides that have one or the other function in the living body. Image reference: www.docbrown.info
Lipids
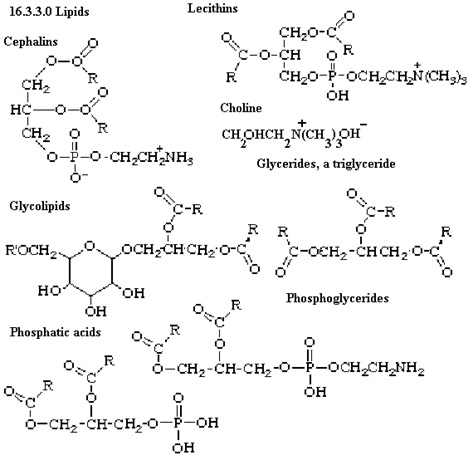 These are organic solvent that are insoluble in water and soluble in organic solvent thus providing structure to the cell. They can be simple or complex. Simple lipids are those that are made of esters of fatty acid and alcohol. Example include: fats, oil and waxes. Likewise, complex lipids are also esters of fatty acid with alcohol but along with this it also has many other groups attached to it like phosphate, carbohydrate etc. These lipids include: phospholipids, glycolipids, lipoproteins etc. Image reference: www.uq.edu.au
These are organic solvent that are insoluble in water and soluble in organic solvent thus providing structure to the cell. They can be simple or complex. Simple lipids are those that are made of esters of fatty acid and alcohol. Example include: fats, oil and waxes. Likewise, complex lipids are also esters of fatty acid with alcohol but along with this it also has many other groups attached to it like phosphate, carbohydrate etc. These lipids include: phospholipids, glycolipids, lipoproteins etc. Image reference: www.uq.edu.au
Proteins
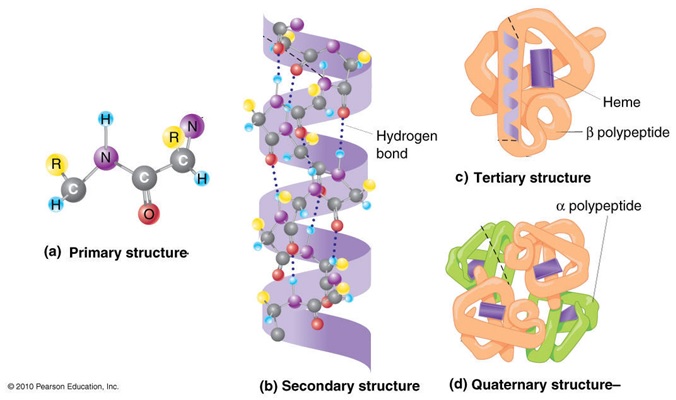 Proteins are also important organic molecules that are fundamental basis of structure and function of life. They are made of chain of amino acid that is linked together with the peptide bond. These amino acid have carbon that is attached to one amino group, one carboxyl group. When pH is higher or lowered i.e. less than 4 or above 9, electrons tends to migrate in the field thus getting added in one or being subtracted from the other. But at pH between 4 and 8, electrons are almost stable and do not migrate. This can be termed as dipolar ion or zwitter ion because they carry both positive and negative charge. Protein can be classified into different types as per their structure called primary structure, secondary structure and tertiary structure.
Proteins are also important organic molecules that are fundamental basis of structure and function of life. They are made of chain of amino acid that is linked together with the peptide bond. These amino acid have carbon that is attached to one amino group, one carboxyl group. When pH is higher or lowered i.e. less than 4 or above 9, electrons tends to migrate in the field thus getting added in one or being subtracted from the other. But at pH between 4 and 8, electrons are almost stable and do not migrate. This can be termed as dipolar ion or zwitter ion because they carry both positive and negative charge. Protein can be classified into different types as per their structure called primary structure, secondary structure and tertiary structure.
Primary structure: In primary configuration, two or more amino acid are held by the peptide linkages where there is free amino end at the left side of the peptide linkage and free carboxyl end at the right side of the linkage. Thus it has linear sequence that forms the backbone of the primary structure.
Secondary structure: It is the spatial arrangement of amino acid by twisting the polypeptide chain. It is of two types: alpha helix and beta sheet. Alpha helix has a rigid arrangement with amino acid side chain extending outward from the central axis. It is well stabilized by hydrogen bonding and all the peptide bond except the corners one or last two that lies in two sides, all other participate in the hydrogen bonding. Likewise, in case of beta sheets, two or more segments of fully extended peptide chains are present and hydrogen bonding takes place between the neighboring segments. The chain can be arranged either in parallel or anti parallel form.
Tertiary structure: It is three dimensional arrangement of protein where hydrophobic side chains lies on the interior of the molecule whereas hydrophilic groups lies on the surface of the protein. It have hydrogen boding along with ionic interaction, disulphide bonds etc.
Quaternary structure: In this case, two or more unidentified protein is present that are held by non- covalent bond and helps in cellular metabolism. Image reference: www.mun.ca
Nucleic acid
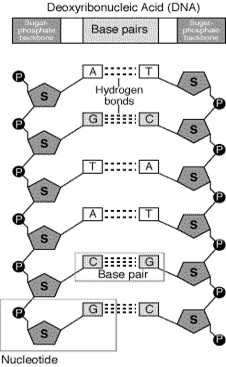 When we talk about nucleic acid, two important names strikes our mind first which are DNA and RNA. These are two important nucleic acid that are found in the living body. DNA called deoxyribonucleic acid and RNA called ribo nucleic acid. DNA consist of two strands or chain of nucleotides that are wound around each other in anti- parallel manner. These strands of DNA are held by hydrogen bonding and the bases that keep these strands close together. These strands are well stabilized by sugar phosphate backbone, where the base linked to sugar is called nucleoside and other that is linked to sugar along with phosphate is called nucleotide. The sugar here is called 2- deoxyribose and are joined by phosphate group resulting in phosphodiester bond. Both these strands of DNA are held anti- parallel, where one strand runs from 3- 5 direction and the other runs from 5- 3 direction. RNA is also similar to DNA with some of the basic differences. Here the sugar present is ribose sugar. Image reference: www.contexo.info
When we talk about nucleic acid, two important names strikes our mind first which are DNA and RNA. These are two important nucleic acid that are found in the living body. DNA called deoxyribonucleic acid and RNA called ribo nucleic acid. DNA consist of two strands or chain of nucleotides that are wound around each other in anti- parallel manner. These strands of DNA are held by hydrogen bonding and the bases that keep these strands close together. These strands are well stabilized by sugar phosphate backbone, where the base linked to sugar is called nucleoside and other that is linked to sugar along with phosphate is called nucleotide. The sugar here is called 2- deoxyribose and are joined by phosphate group resulting in phosphodiester bond. Both these strands of DNA are held anti- parallel, where one strand runs from 3- 5 direction and the other runs from 5- 3 direction. RNA is also similar to DNA with some of the basic differences. Here the sugar present is ribose sugar. Image reference: www.contexo.info
DNA has two groups of bases called purines and pyrimidines where purines of one strand always pairs with pyrimidines of the other and thus vice-versa. Here purines means adenine and thymine and pyrimides means cytosine and guanine. In case of RNA thyamine is replaced by uracil.
This way the structure of DNA and RNA carries several functions of the living body along with various processes of replication, transcription and translation, thus producing a protein product that tare essential for several of the purposes.


