Concept of Atoms and Molecules
Atom: The smallest particle of an element that takes part in chemical reaction.
Daltonapos;s Atomic Theory
The main postulates of this atomic theory are
(i)Matter is discrete (i.e., discontinuous) and is made up of atoms. An atom is the smallest (chemically) indivisible particle of an element, which can take part in a chemical reaction.
(ii) Atoms of different elements have different properties and different weights.
(iii) Atoms cannot be created or destroyed. So a chemical reaction is nothing but a rearrangement of atoms and the same number of atoms must be present before and after the reaction.
 (iv) A compound is formed by the union of atoms of one element with atoms of another in a fixed ratio of small whole numbers (1 : 1, 1 : 2, 2 : 3 etc).
(iv) A compound is formed by the union of atoms of one element with atoms of another in a fixed ratio of small whole numbers (1 : 1, 1 : 2, 2 : 3 etc).
All the postulates of Daltonapos;s atomic theory have been proved to be incorrect. An atom is divisible in the sense that it has a structure. Sub-atomic particle are known as electron, proton and neutron.
Atomic Weight
An atom is so minute that it cannot be detected even with the most powerful microscope, let alone placed on a balance pan and weighed. So there is no question of determining the absolute weight of an atom. So chemists decided to determine the relative masses of atoms (i.e., how many times one atom of an element is heavier than another). Hydrogen atom was first selected as standard. The modern reference standard for atomic weights is carbon isotope of mass number 12.
Atomic weight of an element = 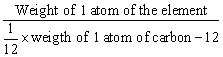
Nowadays atomic weight is called relative atomic mass and denoted by amu
(atomic mass unit). The standard for atomic mass is C12.
(i) Atomic weight is not a weight but a number.
(ii) Atomic weight is not absolute but relative to the weight of the standard reference element (C12).
(iii) Gram atomic weight is atomic weight expressed in grams, but it has a special significance with reference to a mole.
Concept of Atoms and Molecules Assignment Help By Online Tutoring and Guided Sessions at AssignmentHelp.Net
Molecule
The term molecule means the smallest particle of an element or a compound that can exist free and retain all its properties.
Consider a molecule of sulphur dioxide. It has been established that it contains one atom of sulphur and two atoms of oxygen. This molecule can split up into atoms of sulphur and oxygen. So the smallest particle of sulphur dioxide that can exist free and retain all its properties is the molecule of sulphur dioxide.
Molecular Mass
Molecular mass of a substance is an additive property and can be calculated by adding the atomic masses of all the atoms of different elements present in one molecule.
Avogadro Hypothesis
It states that equal volumes of gases at the same temperature and pressure contain equal number of molecules. It means that 1 ml of hydrogen, oxygen, ammonia, or a mixture of gases taken at the same temperature and pressure contains the same number of molecules.
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Stoichiometry chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Stoichiometry Chemistry. Our service is focused on: time delivery, superior quality, creativity, originality and plagiarism free.
To submit chemistry Concept of Atoms and Molecules assignment click here.


