Boyles's Law Assignment Help
Boyle's Law
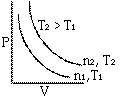
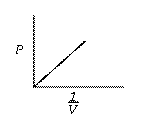
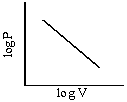
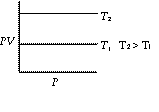
At constant temperature the volume of a given mass of a gas is inversely proportional to its pressure.
Mathematically,
![]() or PV = constant (K), where K depends on mass, temperature and nature of gas.
or PV = constant (K), where K depends on mass, temperature and nature of gas.

![]() when n & T = constant.
when n & T = constant.
Graphs of V vs P or PV vsZ P at constant temperature are known as isotherms.
Boyle's Law Assignment Help By Online Tutoring and Guided Sessions from AssignmentHelp.Net
Homework Help For Boyle's Law
Assignmenthelp.net provides best Online Assignment Help service in Boyle's Law for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Boyle's Law assignment click here


